Mercado europeo de aloinjertos y xenoinjertos espinales, por tipo de producto (aloinjerto, xenoinjerto, suplementos de injerto óseo), abordajes (fusión intercorporal lumbar anterior (ALIF), fusión intercorporal lumbar transforaminal (TLIF), fusión intercorporal lumbar posterior (PLIF), tipo de cirugía (cirugía de columna abierta y cirugía de columna mínimamente invasiva), indicación (enfermedades degenerativas, traumatismos o fracturas espinales, tumores espinales, cirugías de revisión, infecciones espinales (osteomielitis o discitis), deformidades espinales, anomalías espinales congénitas y otras), grupo de edad (adultos, geriátricos y pediátricos), usuario final (hospital, clínica especializada, centros quirúrgicos ambulatorios y otros) - Tendencias de la industria y pronóstico hasta 2030.
Análisis y tamaño del mercado de aloinjertos y xenoinjertos espinales en Europa
Europa tiene una población que envejece significativamente y las afecciones de la columna vertebral, como la enfermedad degenerativa del disco y las fracturas de columna, se vuelven más comunes con la edad. A medida que aumenta la población de edad avanzada, existe una mayor demanda de procedimientos e injertos de columna, lo que impulsa el crecimiento del mercado. Los trastornos de la columna vertebral, incluida la enfermedad degenerativa del disco, la estenosis espinal y las fracturas vertebrales, son relativamente comunes en Europa. Estas afecciones a menudo requieren injertos espinales como parte de los tratamientos quirúrgicos, lo que contribuye al crecimiento del mercado. Los avances en las técnicas de cirugía de columna, como los procedimientos mínimamente invasivos y la cirugía asistida por robot, han aumentado la demanda de materiales de injerto que puedan respaldar estos enfoques innovadores.
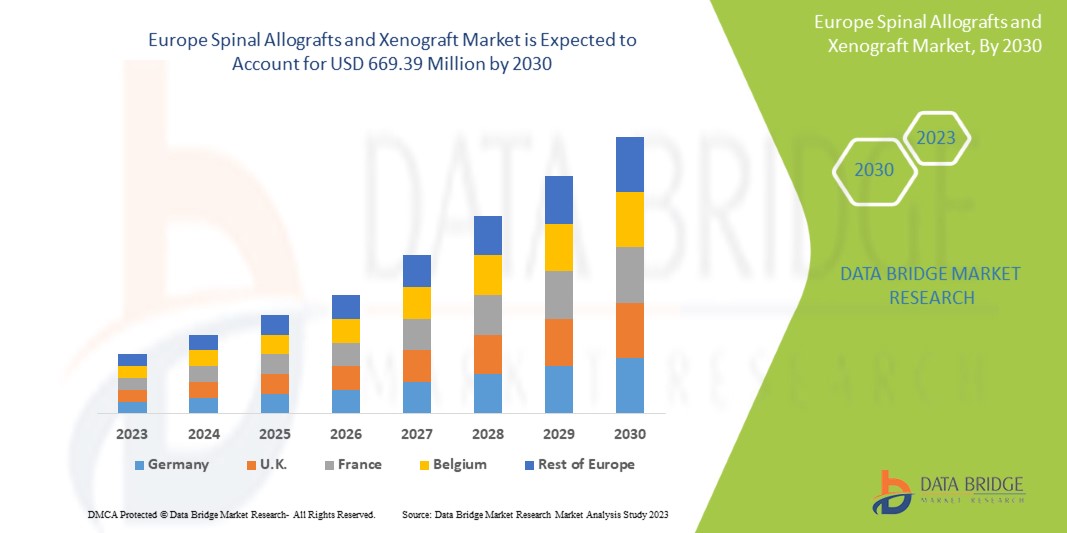
Data Bridge Market Research analiza que se espera que el mercado europeo de aloinjertos y xenoinjertos espinales alcance un valor de USD 669,39 millones para 2030, con una CAGR del 5,6 % durante el período de pronóstico. Este informe de mercado también cubre en profundidad el análisis de precios y los avances tecnológicos.
|
Métrica del informe |
Detalles |
|
Período de pronóstico |
2023 a 2030 |
|
Año base |
2022 |
|
Años históricos |
2021 (Personalizable para 2015 - 2020) |
|
Unidades cuantitativas |
Ingresos en millones de USD |
|
Segmentos cubiertos |
Tipo de producto ( aloinjerto , xenoinjerto, suplementos de injerto óseo), abordajes (fusión intercorporal lumbar anterior [ALIF], fusión intercorporal lumbar transforaminal [TLIF], fusión intercorporal lumbar posterior [PLIF]), tipo de cirugía (cirugía de columna abierta y cirugía de columna mínimamente invasiva), indicación (enfermedades degenerativas, traumatismos o fracturas de columna, tumores de columna, cirugías de revisión, infecciones de columna (osteomielitis o discitis), deformidades de columna, anomalías congénitas de columna y otras), grupo de edad (adultos, geriátricos y pediátricos), usuario final (hospital, clínica especializada, centros quirúrgicos ambulatorios y otros) |
|
Países cubiertos |
Alemania, Francia, Reino Unido, Italia, España, Rusia, Suiza, Países Bajos, Turquía, Polonia, Suecia, Bélgica, Dinamarca, Finlandia, Noruega y resto de Europa. |
|
Actores del mercado cubiertos |
Medtronic, Arthrex, Inc., Stryker, ZimVie Inc., Medical Devices Business Services, Inc., RTI Surgical, Integra LifeSciences, Orthofix US LLC., ATEC Spine, Inc, Globus Medical, Exactech, Inc., Regenity, Cerapedics.Inc, Bioventus y otros. |
Definición de mercado
Un aloinjerto espinal es un material de injerto biológico utilizado en cirugía de columna. Se obtiene de un donante humano, generalmente un cadáver, y se procesa para eliminar células y antígenos, lo que reduce el riesgo de rechazo del injerto y transmisión de enfermedades. El hueso del aloinjerto procesado se utiliza para promover el crecimiento y la fusión ósea en procedimientos espinales, como cirugías de fusión espinal, para tratar diversas afecciones espinales. Un xenoinjerto espinal es un material de injerto utilizado en cirugía espinal, proveniente de una especie no humana, generalmente un animal como una vaca (xenoinjerto bovino) o un cerdo (xenoinjerto porcino). Los xenoinjertos se procesan para reducir el riesgo de rechazo inmunológico y transmisión de enfermedades. Se emplean en cirugías espinales como una alternativa a los aloinjertos humanos cuando no hay donantes humanos adecuados disponibles o cuando el cirujano y el paciente prefieren una fuente de hueso no humano. El mercado europeo de aloinjertos y xenoinjertos espinales se refiere al segmento de la industria médica que involucra la producción, distribución y utilización de materiales de aloinjerto y xenoinjerto espinal para procedimientos quirúrgicos relacionados con la columna.
Dinámica del mercado de aloinjertos y xenoinjertos espinales en Europa
En esta sección se aborda la comprensión de los factores impulsores, las ventajas, las oportunidades, las limitaciones y los desafíos del mercado. Todo esto se analiza en detalle a continuación:
Conductores
- Aumento de la incidencia de trastornos de la columna vertebral
Los trastornos de la columna vertebral abarcan una amplia gama de afecciones que afectan la columna vertebral, la médula espinal y las estructuras asociadas. Varios factores contribuyen a la creciente incidencia de los trastornos de la columna vertebral y, a su vez, a la demanda de materiales para injertos espinales. Los estilos de vida modernos, que pueden implicar estar mucho tiempo sentado, una actividad física reducida y una mala postura, pueden contribuir al desarrollo de trastornos de la columna vertebral. Estos factores pueden acelerar el desgaste de las estructuras espinales y aumentar el riesgo de afecciones como hernias discales y degeneración lumbar. Las lesiones traumáticas, como los accidentes automovilísticos, las caídas y las lesiones relacionadas con los deportes, pueden provocar fracturas espinales, dislocaciones y otros trastornos espinales graves. Estas lesiones a menudo requieren intervenciones quirúrgicas y el uso de materiales de injerto para la reconstrucción espinal.
- Avances en las técnicas quirúrgicas utilizadas en el injerto espinal
La integración de la robótica en la cirugía de columna ha mejorado la precisión, exactitud y resultados quirúrgicos. Los cirujanos pueden realizar procedimientos complejos con mayor confianza. Las cirugías asistidas por robot a menudo requieren materiales de injerto avanzados que se puedan utilizar junto con sistemas robóticos. Esto impulsa la necesidad de injertos compatibles con la cirugía robótica, lo que contribuye a la expansión del mercado. Los sistemas de navegación y las herramientas de imágenes intraoperatorias ayudan a los cirujanos a planificar y ejecutar procedimientos de columna de manera más eficaz. La navegación precisa ayuda a los cirujanos a determinar la colocación óptima de los materiales de injerto, lo que mejora su eficacia para promover la fusión y la estabilidad espinal. Los avances en ingeniería de tejidos y medicina regenerativa han abierto nuevas posibilidades para desarrollar materiales de injerto con biocompatibilidad mejorada y propiedades de fusión. La aparición de materiales de injerto innovadores, como construcciones de ingeniería de tejidos e injertos biológicamente activos, ofrece mejores opciones para los cirujanos de columna y los pacientes, lo que se espera que impulse el crecimiento del mercado.
Oportunidad
- Aumentar la concienciación entre los pacientes sobre los beneficios de los injertos
Los pacientes están cada vez más dispuestos a considerar intervenciones quirúrgicas basadas en injertos. Los pacientes informados tienen más probabilidades de participar activamente en el proceso de toma de decisiones sobre su atención médica. Los pacientes que comprenden los beneficios de los aloinjertos y xenoinjertos pueden ser más receptivos a los procedimientos quirúrgicos que involucran estos materiales, lo que aumenta la demanda de cirugías basadas en injertos. Los pacientes buscan cada vez más tratamientos que ofrezcan mejores resultados, una recuperación más rápida y menos dolor. Los materiales de injerto son fundamentales para lograr la fusión espinal y la estabilización ortopédica, lo que puede conducir a mejores resultados para el paciente. Es más probable que los pacientes soliciten cirugías basadas en injertos para lograr estos beneficios. A menudo, los pacientes se sienten motivados a elegir procedimientos que resulten en una reducción del dolor posoperatorio y una recuperación más rápida. Los materiales de injerto desempeñan un papel crucial en la fusión espinal y la estabilización, lo que contribuye a tiempos de recuperación más cortos y menos dolor. La preferencia de los pacientes por estos beneficios puede impulsar la demanda de procedimientos basados en injertos.
La creciente conciencia de los pacientes sobre las ventajas de los injertos en las cirugías de columna y ortopédicas está creando oportunidades para el crecimiento del mercado. Los pacientes que están bien informados sobre estos beneficios tienen más probabilidades de considerar las intervenciones quirúrgicas basadas en injertos, lo que genera una mayor demanda de materiales y procedimientos para injertos.
Restricción / Desafío
- Riesgo de complicaciones relacionadas con el injerto espinal
Si bien estos materiales de injerto son esenciales para lograr la fusión y estabilidad espinal, las complicaciones asociadas con su uso pueden tener implicaciones tanto para los pacientes como para los proveedores de atención médica. En algunos casos, el sistema inmunológico del receptor puede reconocer el material del injerto como tejido extraño y generar una respuesta inmunológica, lo que lleva al rechazo del injerto. El riesgo de rechazo del injerto puede disuadir a los cirujanos y pacientes de usar aloinjertos y xenoinjertos, lo que lleva a una preferencia por opciones sintéticas o de autoinjerto. La infección es una complicación potencial de cualquier procedimiento quirúrgico, incluidas las cirugías espinales. Los materiales de injerto pueden servir como un sitio para el desarrollo de una infección. El riesgo de infección posoperatoria puede hacer que los proveedores de atención médica y los pacientes sean cautelosos sobre el uso de materiales de injerto, lo que afecta el crecimiento del mercado. Las complicaciones o problemas con la integración del injerto pueden llevar a una fusión espinal retrasada o fallida, lo que requiere cirugías adicionales. Los cirujanos y los pacientes pueden estar preocupados por la posibilidad de una fusión retrasada, lo que lleva a un enfoque cauteloso a la selección del material de injerto.
Los proveedores de atención médica priorizan las evaluaciones de los pacientes, la planificación preoperatoria y la atención posoperatoria para minimizar las complicaciones asociadas con los aloinjertos y xenoinjertos espinales y abordar estos riesgos. Además, los esfuerzos de I+D en curso se centran en mejorar los materiales de los injertos y las técnicas quirúrgicas para mejorar la seguridad y los resultados del paciente.
Por lo tanto, se espera que el riesgo de complicaciones relacionadas con los injertos espinales restrinja el crecimiento del mercado.
Alcance del mercado de aloinjertos y xenoinjertos espinales en Europa
El mercado europeo de aloinjertos y xenoinjertos espinales se divide en seis segmentos importantes según el tipo de producto, los métodos, el tipo de cirugía, la indicación, el grupo de edad y el usuario final. El crecimiento entre estos segmentos le ayudará a analizar los segmentos de crecimiento escaso en las industrias y brindará a los usuarios una valiosa descripción general del mercado y conocimientos del mercado para ayudarlos a tomar decisiones estratégicas para identificar las principales aplicaciones del mercado.
Tipo de producto
- Aloinjerto
- Xenoinjerto
- Suplementos para injertos óseos
Según el tipo de producto, el mercado europeo de aloinjertos y xenoinjertos espinales está segmentado en aloinjertos, xenoinjertos y suplementos de injerto óseo.
Aproches
- Fusión intercorporal lumbar anterior (ALIF)
- Fusión intercorporal lumbar transforaminal (TLIF)
- Fusión intercorporal lumbar posterior (PLIF)
Sobre la base de los enfoques, el mercado europeo de aloinjertos y xenoinjertos espinales se segmenta en fusión intercorporal lumbar anterior (ALIF), fusión intercorporal lumbar transforaminal (TLIF) y fusión intercorporal lumbar posterior (PLIF).
Tipo de cirugía
- Cirugía de columna abierta
- Cirugía de columna mínimamente invasiva
Según el tipo de cirugía, el mercado europeo de aloinjertos y xenoinjertos de columna está segmentado en cirugía de columna abierta y cirugía de columna mínimamente invasiva.
Indicación
- Enfermedades degenerativas
- Traumatismos o fracturas de la columna vertebral
- Tumores de la columna vertebral
- Cirugías de revisión
- Infecciones de la columna ( osteomielitis o discitis)
- Deformidades de la columna vertebral
- Anomalías congénitas de la columna vertebral
- Otros
Sobre la base de la indicación, el mercado europeo de aloinjertos y xenoinjertos espinales está segmentado en enfermedades degenerativas, traumatismos o fracturas espinales, tumores espinales, cirugías de revisión, infecciones espinales (osteomielitis o discitis), deformidades espinales, anomalías espinales congénitas y otros.
Grupo de edad
- Adulto
- Geriátrico
- Pediátrico
Según el grupo de edad, el mercado europeo de aloinjertos y xenoinjertos espinales está segmentado en adultos, geriátricos y pediátricos.
Usuario final
- Hospital
- Clínica de especialidades
- Centros de cirugía ambulatoria
- Otros
Sobre la base del usuario final, el mercado europeo de aloinjertos y xenoinjertos espinales está segmentado en hospitales, clínicas especializadas, centros quirúrgicos ambulatorios y otros.
Análisis y perspectivas regionales del mercado de aloinjertos y xenoinjertos espinales en Europa
El mercado europeo de aloinjertos y xenoinjertos espinales está segmentado en seis segmentos notables según el tipo de producto, los enfoques, el tipo de cirugía, la indicación, el grupo de edad y el usuario final.
Los países cubiertos en este informe de mercado son Alemania, Francia, Reino Unido, Italia, España, Rusia, Suiza, Países Bajos, Turquía, Polonia, Suecia, Bélgica, Dinamarca, Finlandia, Noruega y el resto de Europa.
Se espera que Alemania domine el mercado con la mayor participación de mercado debido a la creciente demanda de injertos espinales.
La sección de países del informe también proporciona factores individuales que impactan en el mercado y cambios en la regulación del mercado que afectan las tendencias actuales y futuras del mercado. Los puntos de datos como el análisis de la cadena de valor ascendente y descendente, las tendencias técnicas, el análisis de las cinco fuerzas de Porter y los estudios de casos son algunos de los indicadores utilizados para pronosticar el escenario del mercado para países individuales. Además, la presencia y disponibilidad de marcas europeas y los desafíos que enfrentan debido a la competencia grande o escasa de las marcas locales y nacionales, el impacto de los aranceles nacionales y las rutas comerciales se consideran al proporcionar un análisis de pronóstico de los datos del país.
Análisis del panorama competitivo y de la cuota de mercado de aloinjertos y xenoinjertos espinales en Europa
El panorama competitivo del mercado de aloinjertos y xenoinjertos espinales en Europa proporciona detalles de los competidores. Los detalles incluidos son una descripción general de la empresa, las finanzas de la empresa, los ingresos generados, el potencial de mercado, las nuevas iniciativas de mercado, la presencia global, los sitios e instalaciones de producción, las capacidades de producción, las fortalezas y debilidades de la empresa, el lanzamiento de productos, la amplitud y variedad de productos y el dominio de las aplicaciones. Los puntos de datos anteriores proporcionados solo están relacionados con el enfoque de las empresas en relación con el mercado.
Algunos de los principales actores del mercado que operan en el mercado europeo de aloinjertos y xenoinjertos espinales son Medtronic, Arthrex, Inc., Stryker, ZimVie Inc. y Medical Devices Business Services, Inc., RTI Surgical, Integra LifeSciences, Orthofix US LLC., ATEC Spine, Inc, Globus Medical, Exactech, Inc., Regenity, Cerapedics.Inc, Bioventus, entre otros.
SKU-
Obtenga acceso en línea al informe sobre la primera nube de inteligencia de mercado del mundo
- Panel de análisis de datos interactivo
- Panel de análisis de empresas para oportunidades con alto potencial de crecimiento
- Acceso de analista de investigación para personalización y consultas
- Análisis de la competencia con panel interactivo
- Últimas noticias, actualizaciones y análisis de tendencias
- Aproveche el poder del análisis de referencia para un seguimiento integral de la competencia
Tabla de contenido
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF THE EUROPE SPINAL ALLOGRAFTS AND XENOGRAFT MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATIONS
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 DBMR TRIPOD DATA VALIDATION MODEL
2.5 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.6 MULTIVARIATE MODELLING
2.7 PRODUCT TYPE SEGMENT LIFELINE CURVE
2.8 MARKET END USER COVERAGE GRID
2.9 DBMR MARKET POSITION GRID
2.1 VENDOR SHARE ANALYSIS
2.11 SECONDARY SOURCES
2.12 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL ANALYSIS
4.2 PORTER’S FIVE FORCES
4.3 MARKET DATA ON PRE-IMPLANT SURGERIES, BY COUNTRY
5 EUROPE SPINAL ALLOGRAFTS AND XENOGRAFT MARKET: REGULATIONS
5.1 REGULATION IN EUROPE
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 RISING INCIDENCE OF SPINAL DISORDERS
6.1.2 ADVANCEMENTS IN SURGICAL TECHNIQUES USED IN SPINAL GRAFT
6.1.3 GROWING DEMAND FOR MINIMALLY INVASIVE PROCEDURES
6.2 RESTRAINTS
6.2.1 RISK OF COMPLICATIONS RELATED TO SPINAL GRAFT
6.2.2 REGULATORY CHALLENGES FOR THE APPROVAL OF NEW GRAFT MATERIALS
6.3 OPPORTUNITIES
6.3.1 RISING AWARENESS AMONG PATIENTS ABOUT THE BENEFITS OF GRAFTS
6.3.2 ADVANCEMENTS IN REGENERATIVE MEDICINES
6.4 CHALLENGES
6.4.1 ETHICAL AND SAFETY CONCERNS REGARDING ALLOGRAFT AND XENOGRAFT
6.4.2 HIGH COST ASSOCIATED WITH SPINAL GRAFT PROCEDURES
7 EUROPE SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE
7.1 OVERVIEW
7.2 ALLOGRAFT
7.2.1 TYPE
7.2.1.1 CANCELLOUS ALLOGRAFT
7.2.1.2 CORTICAL ALLOGRAFT
7.2.1.3 DEMINERALIZED BONE MATRIX
7.2.1.4 OTHERS
7.2.2 INDICATION
7.2.2.1 DEGENERATIVE DISEASES
7.2.2.2 SPINAL TRAUMA OR FRACTURES
7.2.2.3 SPINAL TUMORS
7.2.2.4 REVISION SURGERIES
7.2.2.5 SPINAL INFECTIONS (OSTEOMYELITIS OR DISCITIS)
7.2.2.6 SPINAL DEFORMITIES
7.2.2.7 CONGENITAL SPINAL ABNORMALITIES
7.2.2.8 OTHERS
7.2.3 STORAGE
7.2.3.1 FROZEN (LESS THAN 0°C)
7.2.3.2 REFRIGERATED (0°C TO 10°C)
7.3 XENOGRAFT
7.3.1 TYPE
7.3.2 BOVINE
7.3.3 EQUINE
7.3.4 OTHERS
7.3.5 INDICATION
7.3.5.1 DEGENERATIVE DISEASES
7.3.5.2 SPINAL TRAUMA OR FRACTURES
7.3.5.3 SPINAL TUMORS
7.3.5.4 REVISION SURGERIES
7.3.5.5 SPINAL INFECTIONS (OSTEOMYELITIS OR DISCITIS)
7.3.5.6 SPINAL DEFORMITIES
7.3.5.7 CONGENITAL SPINAL ABNORMALITIES
7.3.5.8 OTHERS
7.4 BONE GRAFT SUPPLEMENTS
7.4.1 MESENCHYMAL STEM CELLS (MSCS)
7.4.2 OSTEOGENIC CELLS
7.4.3 GROWTH FACTORS
7.4.4 BONE MORPHOGENETIC PROTEINS (BMPS)
8 EUROPE SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY APPROACHES
8.1 OVERVIEW
8.2 ANTERIOR LUMBAR INTERBODY FUSION (ALIF)
8.3 TRANSFORAMINAL LUMBAR INTERBODY FUSION (TLIF)
8.4 POSTERIOR LUMBAR INTERBODY FUSION (PLIF)
9 EUROPE SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY SURGERY TYPE
9.1 OVERVIEW
9.2 OPEN SPINE SURGERY
9.2.1 PRODUCT TYPE
9.2.1.1 ALLOGRAFT
9.2.1.2 XENOGRAFT
9.2.2 INDICATION
9.2.2.1 DEGENERATIVE DISEASES
9.2.2.2 SPINAL TRAUMA OR FRACTURES
9.2.2.3 SPINAL TUMORS
9.2.2.4 REVISION SURGERIES
9.2.2.5 SPINAL INFECTIONS (OSTEOMYELITIS OR DISCITIS)
9.2.2.6 SPINAL DEFORMITIES
9.2.2.7 CONGENITAL SPINAL ABNORMALITIES
9.2.2.8 OTHERS
9.3 MINIMALLY INVASIVE SPINE SURGERY
9.3.1 PRODUCT TYPE
9.3.1.1 ALLOGRAFT
9.3.1.2 XENOGRAFT
9.3.2 INDICATION
9.3.2.1 DEGENERATIVE DISEASES
9.3.2.2 SPINAL TRAUMA OR FRACTURES
9.3.2.3 SPINAL TUMORS
9.3.2.4 REVISION SURGERIES
9.3.2.5 SPINAL INFECTIONS (OSTEOMYELITIS OR DISCITIS)
9.3.2.6 SPINAL DEFORMITIES
9.3.2.7 CONGENITAL SPINAL ABNORMALITIES
9.3.2.8 OTHERS
10 EUROPE SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION
10.1 OVERVIEW
10.2 DEGENERATIVE DISEASES
10.2.1 ALLOGRAFT
10.2.2 XENOGRAFT
10.3 SPINAL TRAUMA OR FRACTURES
10.3.1 ALLOGRAFT
10.3.2 XENOGRAFT
10.4 SPINAL TUMORS
10.4.1 ALLOGRAFT
10.4.2 XENOGRAFT
10.5 REVISION SURGERIES
10.5.1 ALLOGRAFT
10.5.2 XENOGRAFT
10.6 SPINAL INFECTIONS (OSTEOMYELITIS OR DISCITIS)
10.6.1 ALLOGRAFT
10.6.2 XENOGRAFT
10.7 SPINAL DEFORMITIES
10.7.1 ALLOGRAFT
10.7.2 XENOGRAFT
10.8 CONGENITAL SPINAL ABNORMALITIES
10.8.1 ALLOGRAFT
10.8.2 XENOGRAFT
10.9 OTHERS
11 EUROPE SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY AGE GROUP
11.1 OVERVIEW
11.2 ADULT
11.3 GERIATRIC
11.4 PEDIATRIC
12 EUROPE SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY END USER
12.1 OVERVIEW
12.2 HOSPITALS
12.2.1 TYPE
12.2.1.1 PUBLIC
12.2.1.2 PRIVATE
12.2.2 TIER
12.2.2.1 TIER 1
12.2.2.2 TIER 2
12.2.2.3 TIER 3
12.3 SPECIALTY CLINIC
12.4 AMBULATORY SURGICAL CENTERS
12.5 OTHERS
13 EUROPE SPINAL ALLOGRAFTS AND XENOGRAFT MARKET BY REGION
13.1 EUROPE
13.1.1 GERMANY
13.1.2 FRANCE
13.1.3 U.K.
13.1.4 ITALY
13.1.5 SPAIN
13.1.6 RUSSIA
13.1.7 SWITZERLAND
13.1.8 NETHERLANDS
13.1.9 TURKEY
13.1.10 POLAND
13.1.11 SWEDEN
13.1.12 BELGIUM
13.1.13 DENMARK
13.1.14 FINLAND
13.1.15 NORWAY
13.1.16 REST OF EUROPE
14 EUROPE SPINAL ALLOGRAFTS AND XENOGRAFT MARKET: COMPANY LANDSCAPE
14.1 COMPANY SHARE ANALYSIS: EUROPE
15 SWOT ANALYSIS
16 COMPANY PROFILE
16.1 MEDTRONIC
16.1.1 COMPANY SNAPSHOT
16.1.2 REVENUE ANALYSIS
16.1.3 PRODUCT PORTFOLIO
16.1.4 RECENT DEVELOPMENT
16.2 ARTHREX, INC.
16.2.1 COMPANY SNAPSHOT
16.2.2 PRODUCT PORTFOLIO
16.2.3 RECENT DEVELOPMENT
16.3 STRYKER
16.3.1 COMPANY SNAPSHOT
16.3.2 REVENUE ANALYSIS
16.3.3 PRODUCT PORTFOLIO
16.3.4 RECENT DEVELOPMENT
16.4 ZIMVIE INC.
16.4.1 COMPANY SNAPSHOT
16.4.2 EVENUE ANALYSIS
16.4.3 PRODUCT PORTFOLIO
16.4.4 RECENT DEVELOPMENT
16.5 MEDICAL DEVICES BUSINESS SERVICES, INC.
16.5.1 COMPANY SNAPSHOT
16.5.2 PRODUCT PORTFOLIO
16.5.3 RECENT DEVELOPMENT
16.6 ATEC SPINE, INC
16.6.1 COMPANY SNAPSHOT
16.6.2 REVENUE ANALYSIS
16.6.3 PRODUCT PORTFOLIO
16.6.4 RECENT DEVELOPMENT
16.7 BIOVENTUS
16.7.1 COMPANY SNAPSHOT
16.7.2 REVENUE ANALYSIS
16.7.3 PRODUCT PORTFOLIO
16.7.4 RECENT DEVELOPMENT
16.8 CERAPEDICS.INC
16.8.1 COMPANY SNAPSHOT
16.8.2 PRODUCT PORTFOLIO
16.8.3 RECENT DEVELOPMENT
16.9 EXACTECH, INC.
16.9.1 COMPANY SNAPSHOT
16.9.2 PRODUCT PORTFOLIO
16.9.3 RECENT DEVELOPMENT
16.1 GLOBUS MEDICAL
16.10.1 COMPANY SNAPSHOT
16.10.2 REVENUE ANALYSIS
16.10.3 PRODUCT PORTFOLIO
16.10.4 RECENT DEVELOPMENT
16.11 INTEGRA LIFESCIENCES
16.11.1 COMPANY SNAPSHOT
16.11.2 REVENUE ANALYSIS
16.11.3 PRODUCT PORTFOLIO
16.11.4 RECENT DEVELOPMENT
16.12 ORTHOFIX US LLC.
16.12.1 COMPANY SNAPSHOT
16.12.2 REVENUE ANALYSIS
16.12.3 PRODUCT PORTFOLIO
16.12.4 RECENT DEVELOPMENT
16.13 REGENITY
16.13.1 COMPANY SNAPSHOT
16.13.2 PRODUCT PORTFOLIO
16.13.3 RECENT DEVELOPMENT
16.14 RTI SURGICAL
16.14.1 COMPANY SNAPSHOT
16.14.2 PRODUCT PORTFOLIO
16.14.3 RECENT DEVELOPMENT
17 QUESTIONNAIRE
18 RELATED REPORTS
Lista de Tablas
TABLE 1 MARKET DATA OF MAXILLOFACIAL SURGERIES
TABLE 2 MARKET DATA OF ORTHOPEDIC SURGERIES
TABLE 3 MARKET DATA OF NUMBER OF DENTAL SURGERIES
TABLE 4 EUROPE SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 5 EUROPE ALLOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 6 EUROPE ALLOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 7 EUROPE ALLOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY STORAGE, 2021-2030 (USD MILLION)
TABLE 8 EUROPE XENOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 9 EUROPE XENOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 10 EUROPE BONE GRAFT SUPPLEMENTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TYPE, 2021-2030 (USD MILLION))
TABLE 11 EUROPE SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY APPROACHES, 2021-2030 (USD MILLION)
TABLE 12 EUROPE SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY SURGERY TYPE , 2021-2030 (USD MILLION )
TABLE 13 EUROPE OPEN SPINE SURGERY IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 14 EUROPE OPEN SPINE SURGERY IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 15 EUROPE MINIMALLY INVASIVE SPINE SURGERY IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 16 EUROPE MINIMALLY INVASIVE SPINE SURGERY IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 17 EUROPE SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 18 EUROPE DEGENERATIVE DISEASES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 19 EUROPE SPINAL TRAUMA OR FRACTURES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 20 EUROPE SPINAL TUMORS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 21 EUROPE REVISION SURGERIES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 22 EUROPE SPINAL INFECTIONS (OSTEOMYELITIS OR DISCITIS) IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 23 EUROPE SPINAL DEFORMITIES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 24 EUROPE CONGENITAL SPINAL ABNORMALITIES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 25 EUROPE SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 26 EUROPE SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 27 EUROPE HOSPITAL IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 28 EUROPE HOSPITAL IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TIER, 2021-2030 (USD MILLION)
TABLE 29 EUROPE SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY COUNTRY, 2021-2030 (USD MILLION)
TABLE 30 GERMANY SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 31 GERMANY ALLOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 32 GERMANY ALLOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 33 GERMANY ALLOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY STORAGE, 2021-2030 (USD MILLION)
TABLE 34 GERMANY XENOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 35 GERMANY XENOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 36 GERMANY BONE GRAFT SUPPLEMENTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 37 GERMANY SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY APPROACHES, 2021-2030 (USD MILLION)
TABLE 38 GERMANY SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY SURGERY TYPE, 2021-2030 (USD MILLION)
TABLE 39 GERMANY OPEN SPINE SURGERY IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 40 GERMANY OPEN SPINE SURGERY IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 41 GERMANY MINIMALLY INVASIVE SPINE SURGERY IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 42 GERMANY MINIMALLY INVASIVE SPINE SURGERY IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 43 GERMANY SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 44 GERMANY DEGENERATIVE DISEASES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 45 GERMANY SPINAL TRAUMA OR FRACTURES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 46 GERMANY SPINAL TUMORS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 47 GERMANY REVISION SURGERIES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 48 GERMANY SPINAL INFECTIONS (OSTEOMYELITIS OR DISCITIS) IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 49 GERMANY SPINAL DEFORMITIES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 50 GERMANY CONGENITAL SPINAL ABNORMALITIES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 51 GERMANY SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 52 GERMANY SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 53 GERMANY HOSPITAL IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 54 GERMANY HOSPITAL IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TIER, 2021-2030 (USD MILLION)
TABLE 55 FRANCE SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 56 FRANCE ALLOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 57 FRANCE ALLOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 58 FRANCE ALLOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY STORAGE, 2021-2030 (USD MILLION)
TABLE 59 FRANCE XENOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 60 FRANCE XENOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 61 FRANCE BONE GRAFT SUPPLEMENTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 62 FRANCE SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY APPROACHES, 2021-2030 (USD MILLION)
TABLE 63 FRANCE SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY SURGERY TYPE, 2021-2030 (USD MILLION)
TABLE 64 FRANCE OPEN SPINE SURGERY IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 65 FRANCE OPEN SPINE SURGERY IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 66 FRANCE MINIMALLY INVASIVE SPINE SURGERY IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 67 FRANCE MINIMALLY INVASIVE SPINE SURGERY IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 68 FRANCE SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 69 FRANCE DEGENERATIVE DISEASES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 70 FRANCE SPINAL TRAUMA OR FRACTURES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 71 FRANCE SPINAL TUMORS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 72 FRANCE REVISION SURGERIES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 73 FRANCE SPINAL INFECTIONS (OSTEOMYELITIS OR DISCITIS) IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 74 FRANCE SPINAL DEFORMITIES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 75 FRANCE CONGENITAL SPINAL ABNORMALITIES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 76 FRANCE SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY AGE GROUP , 2021-2030 (USD MILLION)
TABLE 77 FRANCE SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY END USER , 2021-2030 (USD MILLION)
TABLE 78 FRANCE HOSPITAL IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 79 FRANCE HOSPITAL IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TIER, 2021-2030 (USD MILLION)
TABLE 80 U.K. SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 81 U.K. ALLOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 82 U.K. ALLOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 83 U.K. ALLOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY STORAGE, 2021-2030 (USD MILLION)
TABLE 84 U.K. XENOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 85 U.K. XENOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 86 U.K. BONE GRAFT SUPPLEMENTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 87 U.K. SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY APPROACHES, 2021-2030 (USD MILLION)
TABLE 88 U.K. SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY SURGERY TYPE, 2021-2030 (USD MILLION)
TABLE 89 U.K. OPEN SPINE SURGERY IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 90 U.K. OPEN SPINE SURGERY IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 91 U.K. MINIMALLY INVASIVE SPINE SURGERY IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 92 U.K. MINIMALLY INVASIVE SPINE SURGERY IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 93 U.K. SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 94 U.K. DEGENERATIVE DISEASES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 95 U.K. SPINAL TRAUMA OR FRACTURES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 96 U.K. SPINAL TUMORS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 97 U.K. REVISION SURGERIES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 98 U.K. SPINAL INFECTIONS (OSTEOMYELITIS OR DISCITIS) IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 99 U.K. SPINAL DEFORMITIES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 100 U.K. CONGENITAL SPINAL ABNORMALITIES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 101 U.K. SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY AGE GROUP , 2021-2030 (USD MILLION)
TABLE 102 U.K. SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 103 U.K. HOSPITAL IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 104 U.K. HOSPITAL IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TIER, 2021-2030 (USD MILLION)
TABLE 105 ITALY SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 106 ITALY ALLOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 107 ITALY ALLOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 108 ITALY ALLOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY STORAGE, 2021-2030 (USD MILLION)
TABLE 109 ITALY XENOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 110 ITALY XENOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 111 ITALY BONE GRAFT SUPPLEMENTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 112 ITALY SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY APPROACHES, 2021-2030 (USD MILLION)
TABLE 113 ITALY SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY SURGERY TYPE, 2021-2030 (USD MILLION)
TABLE 114 ITALY OPEN SPINE SURGERY IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 115 ITALY OPEN SPINE SURGERY IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 116 ITALY MINIMALLY INVASIVE SPINE SURGERY IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 117 ITALY MINIMALLY INVASIVE SPINE SURGERY IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 118 ITALY SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 119 ITALY DEGENERATIVE DISEASES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 120 ITALY SPINAL TRAUMA OR FRACTURES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 121 ITALY SPINAL TUMORS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 122 ITALY REVISION SURGERIES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 123 ITALY SPINAL INFECTIONS (OSTEOMYELITIS OR DISCITIS) IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 124 ITALY SPINAL DEFORMITIES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 125 ITALY CONGENITAL SPINAL ABNORMALITIES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 126 ITALY SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 127 ITALY SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY END USER , 2021-2030 (USD MILLION)
TABLE 128 ITALY HOSPITAL IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 129 ITALY HOSPITAL IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TIER, 2021-2030 (USD MILLION)
TABLE 130 SPAIN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 131 SPAIN ALLOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 132 SPAIN ALLOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 133 SPAIN ALLOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY STORAGE, 2021-2030 (USD MILLION)
TABLE 134 SPAIN XENOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 135 SPAIN XENOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 136 SPAIN BONE GRAFT SUPPLEMENTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 137 SPAIN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY APPROACHES, 2021-2030 (USD MILLION)
TABLE 138 SPAIN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY SURGERY TYPE, 2021-2030 (USD MILLION)
TABLE 139 SPAIN OPEN SPINE SURGERY IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 140 SPAIN OPEN SPINE SURGERY IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 141 SPAIN MINIMALLY INVASIVE SPINE SURGERY IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 142 SPAIN MINIMALLY INVASIVE SPINE SURGERY IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 143 SPAIN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 144 SPAIN DEGENERATIVE DISEASES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 145 SPAIN SPINAL TRAUMA OR FRACTURES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 146 SPAIN SPINAL TUMORS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 147 SPAIN REVISION SURGERIES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 148 SPAIN SPINAL INFECTIONS (OSTEOMYELITIS OR DISCITIS) IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 149 SPAIN SPINAL DEFORMITIES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 150 SPAIN CONGENITAL SPINAL ABNORMALITIES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 151 SPAIN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY AGE GROUP , 2021-2030 (USD MILLION)
TABLE 152 SPAIN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY END USER , 2021-2030 (USD MILLION)
TABLE 153 SPAIN HOSPITAL IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 154 SPAIN HOSPITAL IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TIER, 2021-2030 (USD MILLION)
TABLE 155 RUSSIA SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 156 RUSSIA ALLOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 157 RUSSIA ALLOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 158 RUSSIA ALLOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY STORAGE, 2021-2030 (USD MILLION)
TABLE 159 RUSSIA XENOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 160 RUSSIA XENOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 161 RUSSIA BONE GRAFT SUPPLEMENTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 162 RUSSIA SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY APPROACHES, 2021-2030 (USD MILLION)
TABLE 163 RUSSIA SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY SURGERY TYPE, 2021-2030 (USD MILLION)
TABLE 164 RUSSIA OPEN SPINE SURGERY IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 165 RUSSIA OPEN SPINE SURGERY IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 166 RUSSIA MINIMALLY INVASIVE SPINE SURGERY IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 167 RUSSIA MINIMALLY INVASIVE SPINE SURGERY IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 168 RUSSIA SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 169 RUSSIA DEGENERATIVE DISEASES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 170 RUSSIA SPINAL TRAUMA OR FRACTURES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 171 RUSSIA SPINAL TUMORS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 172 RUSSIA REVISION SURGERIES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 173 RUSSIA SPINAL INFECTIONS (OSTEOMYELITIS OR DISCITIS) IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 174 RUSSIA SPINAL DEFORMITIES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 175 RUSSIA CONGENITAL SPINAL ABNORMALITIES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 176 RUSSIA SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 177 RUSSIA SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 178 RUSSIA HOSPITAL IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 179 RUSSIA HOSPITAL IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TIER, 2021-2030 (USD MILLION)
TABLE 180 SWITZERLAND SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 181 SWITZERLAND ALLOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 182 SWITZERLAND ALLOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 183 SWITZERLAND ALLOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY STORAGE, 2021-2030 (USD MILLION)
TABLE 184 SWITZERLAND XENOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 185 SWITZERLAND XENOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 186 SWITZERLAND BONE GRAFT SUPPLEMENTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 187 SWITZERLAND SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY APPROACHES, 2021-2030 (USD MILLION)
TABLE 188 SWITZERLAND SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY SURGERY TYPE, 2021-2030 (USD MILLION)
TABLE 189 SWITZERLAND OPEN SPINE SURGERY IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 190 SWITZERLAND OPEN SPINE SURGERY IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 191 SWITZERLAND MINIMALLY INVASIVE SPINE SURGERY IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 192 SWITZERLAND MINIMALLY INVASIVE SPINE SURGERY IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 193 SWITZERLAND SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 194 SWITZERLAND DEGENERATIVE DISEASES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 195 SWITZERLAND SPINAL TRAUMA OR FRACTURES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 196 SWITZERLAND SPINAL TUMORS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 197 SWITZERLAND REVISION SURGERIES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 198 SWITZERLAND SPINAL INFECTIONS (OSTEOMYELITIS OR DISCITIS) IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 199 SWITZERLAND SPINAL DEFORMITIES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 200 SWITZERLAND CONGENITAL SPINAL ABNORMALITIES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 201 SWITZERLAND SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY AGE GROUP , 2021-2030 (USD MILLION)
TABLE 202 SWITZERLAND SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 203 SWITZERLAND HOSPITAL IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 204 SWITZERLAND HOSPITAL IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TIER, 2021-2030 (USD MILLION)
TABLE 205 NETHERLANDS SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 206 NETHERLANDS ALLOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 207 NETHERLANDS ALLOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 208 NETHERLANDS ALLOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY STORAGE, 2021-2030 (USD MILLION)
TABLE 209 NETHERLANDS XENOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 210 NETHERLANDS XENOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 211 NETHERLANDS BONE GRAFT SUPPLEMENTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 212 NETHERLANDS SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY APPROACHES , 2021-2030 (USD MILLION)
TABLE 213 NETHERLANDS SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY SURGERY TYPE, 2021-2030 (USD MILLION)
TABLE 214 NETHERLANDS OPEN SPINE SURGERY IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 215 NETHERLANDS OPEN SPINE SURGERY IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 216 NETHERLANDS MINIMALLY INVASIVE SPINE SURGERY IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 217 NETHERLANDS MINIMALLY INVASIVE SPINE SURGERY IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 218 NETHERLANDS SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 219 NETHERLANDS DEGENERATIVE DISEASES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 220 NETHERLANDS SPINAL TRAUMA OR FRACTURES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 221 NETHERLANDS SPINAL TUMORS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 222 NETHERLANDS REVISION SURGERIES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 223 NETHERLANDS SPINAL INFECTIONS (OSTEOMYELITIS OR DISCITIS) IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 224 NETHERLANDS SPINAL DEFORMITIES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 225 NETHERLANDS CONGENITAL SPINAL ABNORMALITIES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 226 NETHERLANDS SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 227 NETHERLANDS SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 228 NETHERLANDS HOSPITAL IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 229 NETHERLANDS HOSPITAL IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TIER, 2021-2030 (USD MILLION)
TABLE 230 TURKEY SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 231 TURKEY ALLOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 232 TURKEY ALLOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 233 TURKEY ALLOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY STORAGE, 2021-2030 (USD MILLION)
TABLE 234 TURKEY XENOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 235 TURKEY XENOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 236 TURKEY BONE GRAFT SUPPLEMENTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 237 TURKEY SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY APPROACHES , 2021-2030 (USD MILLION)
TABLE 238 TURKEY SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY SURGERY TYPE, 2021-2030 (USD MILLION)
TABLE 239 TURKEY OPEN SPINE SURGERY IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 240 TURKEY OPEN SPINE SURGERY IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 241 TURKEY MINIMALLY INVASIVE SPINE SURGERY IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 242 TURKEY MINIMALLY INVASIVE SPINE SURGERY IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 243 TURKEY SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 244 TURKEY DEGENERATIVE DISEASES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 245 TURKEY SPINAL TRAUMA OR FRACTURES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 246 TURKEY SPINAL TUMORS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 247 TURKEY REVISION SURGERIES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 248 TURKEY SPINAL INFECTIONS (OSTEOMYELITIS OR DISCITIS) IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 249 TURKEY SPINAL DEFORMITIES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 250 TURKEY CONGENITAL SPINAL ABNORMALITIES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 251 TURKEY SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 252 TURKEY SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 253 TURKEY HOSPITAL IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 254 TURKEY HOSPITAL IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TIER, 2021-2030 (USD MILLION)
TABLE 255 POLAND SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 256 POLAND ALLOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 257 POLAND ALLOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 258 POLAND ALLOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY STORAGE, 2021-2030 (USD MILLION)
TABLE 259 POLAND XENOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 260 POLAND XENOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 261 POLAND BONE GRAFT SUPPLEMENTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 262 POLAND SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY APPROACHES, 2021-2030 (USD MILLION)
TABLE 263 POLAND SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY SURGERY TYPE, 2021-2030 (USD MILLION)
TABLE 264 POLAND OPEN SPINE SURGERY IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 265 POLAND OPEN SPINE SURGERY IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 266 POLAND MINIMALLY INVASIVE SPINE SURGERY IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 267 POLAND MINIMALLY INVASIVE SPINE SURGERY IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 268 POLAND SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 269 POLAND DEGENERATIVE DISEASES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 270 POLAND SPINAL TRAUMA OR FRACTURES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 271 POLAND SPINAL TUMORS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 272 POLAND REVISION SURGERIES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 273 POLAND SPINAL INFECTIONS (OSTEOMYELITIS OR DISCITIS) IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 274 POLAND SPINAL DEFORMITIES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 275 POLAND CONGENITAL SPINAL ABNORMALITIES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 276 POLAND SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 277 POLAND SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 278 POLAND HOSPITAL IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 279 POLAND HOSPITAL IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TIER, 2021-2030 (USD MILLION)
TABLE 280 SWEDEN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 281 SWEDEN ALLOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 282 SWEDEN ALLOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 283 SWEDEN ALLOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY STORAGE, 2021-2030 (USD MILLION)
TABLE 284 SWEDEN XENOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 285 SWEDEN XENOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 286 SWEDEN BONE GRAFT SUPPLEMENTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 287 SWEDEN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY APPROACHES, 2021-2030 (USD MILLION)
TABLE 288 SWEDEN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY SURGERY TYPE, 2021-2030 (USD MILLION)
TABLE 289 SWEDEN OPEN SPINE SURGERY IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 290 SWEDEN OPEN SPINE SURGERY IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 291 SWEDEN MINIMALLY INVASIVE SPINE SURGERY IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 292 SWEDEN MINIMALLY INVASIVE SPINE SURGERY IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 293 SWEDEN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 294 SWEDEN DEGENERATIVE DISEASES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 295 SWEDEN SPINAL TRAUMA OR FRACTURES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 296 SWEDEN SPINAL TUMORS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 297 SWEDEN REVISION SURGERIES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 298 SWEDEN SPINAL INFECTIONS (OSTEOMYELITIS OR DISCITIS) IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 299 SWEDEN SPINAL DEFORMITIES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 300 SWEDEN CONGENITAL SPINAL ABNORMALITIES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 301 SWEDEN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY AGE GROUP , 2021-2030 (USD MILLION)
TABLE 302 SWEDEN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY END USER , 2021-2030 (USD MILLION)
TABLE 303 SWEDEN HOSPITAL IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 304 SWEDEN HOSPITAL IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TIER, 2021-2030 (USD MILLION)
TABLE 305 BELGIUM SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 306 BELGIUM ALLOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 307 BELGIUM ALLOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 308 BELGIUM ALLOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY STORAGE , 2021-2030 (USD MILLION)
TABLE 309 BELGIUM XENOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 310 BELGIUM XENOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 311 BELGIUM BONE GRAFT SUPPLEMENTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 312 BELGIUM SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY APPROACHES, 2021-2030 (USD MILLION)
TABLE 313 BELGIUM SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY SURGERY TYPE, 2021-2030 (USD MILLION)
TABLE 314 BELGIUM OPEN SPINE SURGERY IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 315 BELGIUM OPEN SPINE SURGERY IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 316 BELGIUM MINIMALLY INVASIVE SPINE SURGERY IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 317 BELGIUM MINIMALLY INVASIVE SPINE SURGERY IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 318 BELGIUM SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 319 BELGIUM DEGENERATIVE DISEASES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 320 BELGIUM SPINAL TRAUMA OR FRACTURES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 321 BELGIUM SPINAL TUMORS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 322 BELGIUM REVISION SURGERIES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 323 BELGIUM SPINAL INFECTIONS (OSTEOMYELITIS OR DISCITIS) IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 324 BELGIUM SPINAL DEFORMITIES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 325 BELGIUM CONGENITAL SPINAL ABNORMALITIES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 326 BELGIUM SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 327 BELGIUM SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 328 BELGIUM HOSPITAL IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 329 BELGIUM HOSPITAL IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TIER, 2021-2030 (USD MILLION)
TABLE 330 DENMARK SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 331 DENMARK ALLOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 332 DENMARK ALLOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 333 DENMARK ALLOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY STORAGE, 2021-2030 (USD MILLION)
TABLE 334 DENMARK XENOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 335 DENMARK XENOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 336 DENMARK BONE GRAFT SUPPLEMENTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 337 DENMARK SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY APPROACHES, 2021-2030 (USD MILLION)
TABLE 338 DENMARK SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY SURGERY TYPE, 2021-2030 (USD MILLION)
TABLE 339 DENMARK OPEN SPINE SURGERY IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 340 DENMARK OPEN SPINE SURGERY IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 341 DENMARK MINIMALLY INVASIVE SPINE SURGERY IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 342 DENMARK MINIMALLY INVASIVE SPINE SURGERY IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 343 DENMARK SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 344 DENMARK DEGENERATIVE DISEASES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 345 DENMARK SPINAL TRAUMA OR FRACTURES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 346 DENMARK SPINAL TUMORS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 347 DENMARK REVISION SURGERIES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 348 DENMARK SPINAL INFECTIONS (OSTEOMYELITIS OR DISCITIS) IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 349 DENMARK SPINAL DEFORMITIES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 350 DENMARK CONGENITAL SPINAL ABNORMALITIES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 351 DENMARK SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 352 DENMARK SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 353 DENMARK HOSPITAL IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 354 DENMARK HOSPITAL IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TIER, 2021-2030 (USD MILLION)
TABLE 355 FINLAND SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 356 FINLAND ALLOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 357 FINLAND ALLOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 358 FINLAND ALLOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY STORAGE, 2021-2030 (USD MILLION)
TABLE 359 FINLAND XENOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 360 FINLAND XENOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 361 FINLAND BONE GRAFT SUPPLEMENTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 362 FINLAND SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY APPROACHES, 2021-2030 (USD MILLION)
TABLE 363 FINLAND SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY SURGERY TYPE, 2021-2030 (USD MILLION)
TABLE 364 FINLAND OPEN SPINE SURGERY IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 365 FINLAND OPEN SPINE SURGERY IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 366 FINLAND MINIMALLY INVASIVE SPINE SURGERY IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 367 FINLAND MINIMALLY INVASIVE SPINE SURGERY IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 368 FINLAND SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 369 FINLAND DEGENERATIVE DISEASES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 370 FINLAND SPINAL TRAUMA OR FRACTURES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 371 FINLAND SPINAL TUMORS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 372 FINLAND REVISION SURGERIES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 373 FINLAND SPINAL INFECTIONS (OSTEOMYELITIS OR DISCITIS) IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 374 FINLAND SPINAL DEFORMITIES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 375 FINLAND CONGENITAL SPINAL ABNORMALITIES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 376 FINLAND SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 377 FINLAND SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 378 FINLAND HOSPITAL IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 379 FINLAND HOSPITAL IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TIER, 2021-2030 (USD MILLION)
TABLE 380 NORWAY SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 381 NORWAY ALLOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 382 NORWAY ALLOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 383 NORWAY ALLOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY STORAGE, 2021-2030 (USD MILLION)
TABLE 384 NORWAY XENOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 385 NORWAY XENOGRAFTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 386 NORWAY BONE GRAFT SUPPLEMENTS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 387 NORWAY SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY APPROACHES, 2021-2030 (USD MILLION)
TABLE 388 NORWAY SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY SURGERY TYPE, 2021-2030 (USD MILLION)
TABLE 389 NORWAY OPEN SPINE SURGERY IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 390 NORWAY OPEN SPINE SURGERY IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 391 NORWAY MINIMALLY INVASIVE SPINE SURGERY IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 392 NORWAY MINIMALLY INVASIVE SPINE SURGERY IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 393 NORWAY SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 394 NORWAY DEGENERATIVE DISEASES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 395 NORWAY SPINAL TRAUMA OR FRACTURES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 396 NORWAY SPINAL TUMORS IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 397 NORWAY REVISION SURGERIES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 398 NORWAY SPINAL INFECTIONS (OSTEOMYELITIS OR DISCITIS) IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 399 NORWAY SPINAL DEFORMITIES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 400 NORWAY CONGENITAL SPINAL ABNORMALITIES IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 401 NORWAY SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY AGE GROUP , 2021-2030 (USD MILLION)
TABLE 402 NORWAY SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 403 NORWAY HOSPITAL IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 404 NORWAY HOSPITAL IN SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY TIER, 2021-2030 (USD MILLION)
TABLE 405 REST OF EUROPE SPINAL ALLOGRAFTS AND XENOGRAFT MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
Lista de figuras
FIGURE 1 EUROPE SPINAL ALLOGRAFTS AND XENOGRAFT MARKET: SEGMENTATION
FIGURE 2 EUROPE SPINAL ALLOGRAFTS AND XENOGRAFT MARKET: DATA TRIANGULATION
FIGURE 3 EUROPE SPINAL ALLOGRAFTS AND XENOGRAFT MARKET: DROC ANALYSIS
FIGURE 4 EUROPE SPINAL ALLOGRAFTS AND XENOGRAFT MARKET: REGIONAL VS COUNTRY MARKET ANALYSIS
FIGURE 5 EUROPE SPINAL ALLOGRAFTS AND XENOGRAFT MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 EUROPE SPINAL ALLOGRAFTS AND XENOGRAFT MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 EUROPE SPINAL ALLOGRAFTS AND XENOGRAFT MARKET: MARKET END USER COVERAGE GRID
FIGURE 8 EUROPE SPINAL ALLOGRAFTS AND XENOGRAFT MARKET: DBMR MARKET POSITION GRID
FIGURE 9 EUROPE SPINAL ALLOGRAFTS AND XENOGRAFT MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 EUROPE SPINAL ALLOGRAFTS AND XENOGRAFT MARKET: SEGMENTATION
FIGURE 11 THE RISING INCIDENCE OF SPINAL DISORDERS IS EXPECTED TO DRIVE THE GROWTH OF THE EUROPE SPINAL ALLOGRAFTS AND XENOGRAFT MARKET FROM 2023 TO 2030
FIGURE 12 THE ALLOGRAFT SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE EUROPE SPINAL ALLOGRAFTS AND XENOGRAFT MARKET IN 2023 AND 2030
FIGURE 13 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE EUROPE SPINAL ALLOGRAFTS AND XENOGRAFT MARKET
FIGURE 14 EUROPE SPINAL ALLOGRAFTS AND XENOGRAFT MARKET: BY PRODUCT TYPE, 2022
FIGURE 15 EUROPE SPINAL ALLOGRAFTS AND XENOGRAFT MARKET: BY APPROACHES, 2022
FIGURE 16 EUROPE SPINAL ALLOGRAFTS AND XENOGRAFT MARKET: BY SURGERY TYPE, 2022
FIGURE 17 EUROPE SPINAL ALLOGRAFTS AND XENOGRAFT MARKET: BY INDICATION, 2022
FIGURE 18 EUROPE SPINAL ALLOGRAFTS AND XENOGRAFT MARKET: BY AGE GROUP, 2022
FIGURE 19 EUROPE SPINAL ALLOGRAFTS AND XENOGRAFT MARKET: BY END USER, 2022
FIGURE 20 EUROPE SPINAL ALLOGRAFTS AND XENOGRAFT MARKET: SNAPSHOT (2022)
FIGURE 21 EUROPE SPINAL ALLOGRAFTS AND XENOGRAFT MARKET: COMPANY SHARE 2022 (%)

Metodología de investigación
La recopilación de datos y el análisis del año base se realizan utilizando módulos de recopilación de datos con muestras de gran tamaño. La etapa incluye la obtención de información de mercado o datos relacionados a través de varias fuentes y estrategias. Incluye el examen y la planificación de todos los datos adquiridos del pasado con antelación. Asimismo, abarca el examen de las inconsistencias de información observadas en diferentes fuentes de información. Los datos de mercado se analizan y estiman utilizando modelos estadísticos y coherentes de mercado. Además, el análisis de la participación de mercado y el análisis de tendencias clave son los principales factores de éxito en el informe de mercado. Para obtener más información, solicite una llamada de un analista o envíe su consulta.
La metodología de investigación clave utilizada por el equipo de investigación de DBMR es la triangulación de datos, que implica la extracción de datos, el análisis del impacto de las variables de datos en el mercado y la validación primaria (experto en la industria). Los modelos de datos incluyen cuadrícula de posicionamiento de proveedores, análisis de línea de tiempo de mercado, descripción general y guía del mercado, cuadrícula de posicionamiento de la empresa, análisis de patentes, análisis de precios, análisis de participación de mercado de la empresa, estándares de medición, análisis global versus regional y de participación de proveedores. Para obtener más información sobre la metodología de investigación, envíe una consulta para hablar con nuestros expertos de la industria.
Personalización disponible
Data Bridge Market Research es líder en investigación formativa avanzada. Nos enorgullecemos de brindar servicios a nuestros clientes existentes y nuevos con datos y análisis que coinciden y se adaptan a sus objetivos. El informe se puede personalizar para incluir análisis de tendencias de precios de marcas objetivo, comprensión del mercado de países adicionales (solicite la lista de países), datos de resultados de ensayos clínicos, revisión de literatura, análisis de mercado renovado y base de productos. El análisis de mercado de competidores objetivo se puede analizar desde análisis basados en tecnología hasta estrategias de cartera de mercado. Podemos agregar tantos competidores sobre los que necesite datos en el formato y estilo de datos que esté buscando. Nuestro equipo de analistas también puede proporcionarle datos en archivos de Excel sin procesar, tablas dinámicas (libro de datos) o puede ayudarlo a crear presentaciones a partir de los conjuntos de datos disponibles en el informe.