Global Centronuclear Myopathies Drug Market
Tamaño del mercado en miles de millones de dólares
Tasa de crecimiento anual compuesta (CAGR) :
% 
 USD
240.66 Million
USD
390.25 Million
2024
2032
USD
240.66 Million
USD
390.25 Million
2024
2032
| 2025 –2032 | |
| USD 240.66 Million | |
| USD 390.25 Million | |
|
|
|
|
Global Centronuclear Myopathies Drug Market Segmentation, By Drug Type (Antisense Oligonucleotides, Enzyme Replacement Therapies, and Others), Route of Administration (Oral, Injectable, and Others), End User (Hospitals, Specialty Clinics, Homecare, and Others), and Distribution Channel (Hospital Pharmacies, Retail Pharmacies, and Online Pharmacies) - Industry Trends and Forecast to 2032
Centronuclear Myopathies Drug Market Size
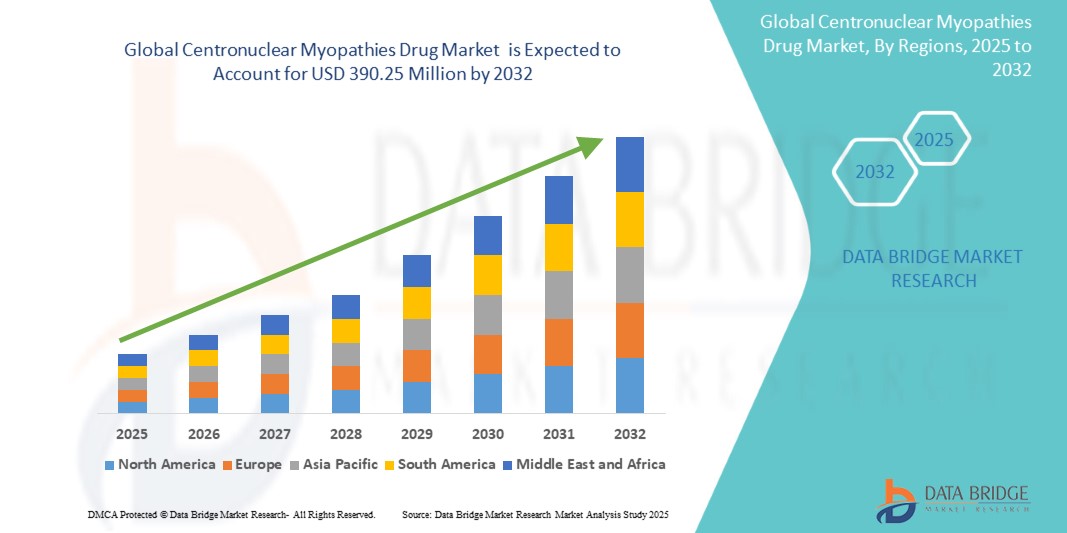
- The Global Centronuclear Myopathies Drug Market size was valued at USD 240.66 million in 2024 and is expected to reach USD 390.25 Million by 2032, at a CAGR of 6.4% during the forecast period
- This growth is driven by increasing R&D activity targeting rare neuromuscular disorders, rising global awareness, and advancements in gene-based and enzyme-targeted therapies.
Centronuclear Myopathies Drug Market Analysis
- Centronuclear myopathies (CNMs) are rare congenital muscle disorders that impair muscle strength and function due to abnormal positioning of cell nuclei. Increasing regulatory support for orphan drugs and early diagnosis through newborn screening are significantly enhancing treatment accessibility and awareness
- The demand for these microscopes is significantly driven by the increasing prevalence of age-related eye conditions and advancements in surgical techniques
- North America is expected to dominate the Centronuclear Myopathies Drugs market due to early access to clinical trials and regulatory approvals
- Asia-Pacific is expected to be the fastest growing region in the Centronuclear Myopathies Drug market during the forecast period due to growth through collaborative research initiatives
- Antisense oligonucleotides and enzyme replacement therapies are gaining traction as the standard of care for certain CNM subtypes, with several candidates in late-phase clinical development
Report Scope and Centronuclear Myopathies Drug Market Segmentation
|
Attributes |
Centronuclear Myopathies Drug Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include import export analysis, production capacity overview, production consumption analysis, price trend analysis, climate change scenario, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Centronuclear Myopathies Drug Market Trends
“Emerging Gene Therapies and Strategic Collaborations”
- One prominent trend in the Centronuclear Myopathies drug market is the rising focus on gene therapies and targeted molecular treatments to address the underlying genetic causes of the disease.
- These innovations, such as Dynacure’s DYN101 and ARMGO Pharma’s ARM210, are being developed through strategic partnerships and offer the potential to significantly improve clinical outcomes by modifying disease progression at the molecular level.
- For instance, in 2024, advancements in high-resolution imaging and next-generation sequencing technologies significantly improved the ability to diagnose Centronuclear Myopathies (CNM) at earlier stages, allowing researchers and clinicians to better understand genotype-phenotype correlations.
- These innovations are transforming the management of CNM by supporting earlier intervention and personalized treatment approaches, thereby driving demand for next-generation therapeutics and targeted drug development.
Centronuclear Myopathies Drug Market Dynamics
Driver
“Advancements in Genetic Therapies and Regulatory Incentives”
- The development of gene therapies and antisense platforms is accelerating treatment availability for CNMs. Regulatory bodies are actively fast-tracking these innovations under orphan drug and rare pediatric designations.
- As advancements in genetic therapies accelerate, particularly with the development of AAV-based gene therapies and exon-skipping techniques, there is growing optimism for disease-modifying treatments for Centronuclear Myopathies (CNM).
- In response, regulatory agencies such as the FDA and EMA have introduced incentives like Orphan Drug Designation and accelerated approval pathways, significantly boosting investment and R&D activity in the CNM drug market
For instance,
- For instance, in 2024, the FDA granted breakthrough therapy status to an antisense candidate developed for X-linked CNM, boosting investor interest and development speed.
- As a result of advancements in genetic therapies and growing regulatory support, there is a significant increase in the development and demand for Centronuclear Myopathies (CNM) drugs, driven by the rising identification of CNM cases through improved genetic screening and increased availability of targeted treatment options
Opportunity
“Increasing Global Patient Advocacy and Awareness Initiatives”
- Rare disease foundations and global neuromuscular alliances are helping spread awareness and influence research priorities. Patient registries and outreach programs have expanded diagnostic reach in underserved regions
- Growing global patient advocacy and awareness initiatives are playing a pivotal role in accelerating early diagnosis and access to treatment for Centronuclear Myopathies (CNM), as organizations work to educate both the public and healthcare professionals about the disease.
- • Additionally, these initiatives are fostering collaboration among researchers, pharmaceutical companies, and regulatory bodies, helping to drive funding, clinical trial participation, and the development of more effective CNM therapies.
For instance,
- For example, in 2025, the Myotubular Trust and European CNM Consortium launched an awareness campaign and biobank platform to centralize patient data and support new therapy trials
- Furthermore, advocacy groups are instrumental in connecting patients with emerging therapies and support networks, empowering families and influencing policy to prioritize rare neuromuscular disease research and treatment access.
Restraint/Challenge
“Limited Access and High Treatment Costs in Low-Income Regions”
- Despite global recognition, many patients in lower-income countries face delays in diagnosis and lack access to treatment due to cost and absence of local expertise.
- Advanced therapies for Centronuclear Myopathies (CNM), such as gene therapies and specialized biologics, often come with high development and distribution costs, making them financially inaccessible for many patients in low-income regions
- This substantial cost barrier limits access to cutting-edge treatments and clinical trials, leading to delayed diagnoses, suboptimal care, and continued reliance on supportive rather than curative therapies in underserved populations.
For instance,
- A 2023 study in The Lancet Neurology reported that 68% of CNM patients in Southeast Asia were undiagnosed or misdiagnosed due to absence of neuromuscular specialists and genetic testing access
- Consequently, such limitations contribute to significant disparities in the diagnosis and treatment of Centronuclear Myopathies (CNM), restricting patient access to emerging genetic therapies and specialized care, and ultimately hindering the global growth and equitable development of the CNM drug market
Centronuclear Myopathies Drug Market Scope
The market is segmented on the basis drug type, route of administration, end user, and distribution channel.
|
Segmentation |
Sub-Segmentation |
|
By Drug Type |
|
|
By Route of Administration |
|
|
By End User |
|
|
By Distribution Channel
|
|
In 2025, the Antisense Oligonucleotides is projected to dominate the market with a largest share in drug type segment
The Antisense Oligonucleotides segment is expected to dominate the Centronuclear Myopathies Drug market with the largest share of 56.22% in 2025 due to its targeted mechanism of action and growing demand for precision medicine in rare neuromuscular disorders. As advancements in genetic research continue, antisense therapies are showing promising clinical outcomes in modifying the expression of mutated genes associated with CNM. Increasing awareness, early diagnosis through genetic screening, and expanding clinical trials further contribute to the segment’s projected market leadership.
The Oral is expected to account for the largest share during the forecast period in technology market
In 2025, the Oral segment is expected to dominate the market with the largest market share of 51.31% due to the convenience of administration, improved patient compliance, and ongoing development of orally delivered small molecules targeting neuromuscular pathways. As research progresses, oral formulations are becoming increasingly viable for managing symptoms and modifying disease progression, particularly in pediatric and adult CNM populations. Additionally, growing demand for non-invasive treatment options supports the segment's projected dominance..
Centronuclear Myopathies Drug Market Regional Analysis
“North America Holds the Largest Share in the Centronuclear Myopathies Drug Market”
- North America is expected to dominate the Centronuclear Myopathies (CNM) drug market, driven by its advanced healthcare infrastructure, high adoption of innovative genetic therapies, and the strong presence of key pharmaceutical and biotechnology companies focused on rare neuromuscular diseases.
- The U.S. holds a significant share due to increasing demand for precision medicine, heightened awareness and diagnosis of CNM through genetic screening, and continuous advancements in gene-based treatments.
- The availability of well-established reimbursement policies for rare disease treatments and significant investments in research & development by leading pharmaceutical companies further strengthen the market.
- Additionally, the growing emphasis on rare disease therapies and the rising number of clinical trials dedicated to CNM is fueling market growth across the region
“Asia-Pacific is Projected to Register the Highest CAGR in the Centronuclear Myopathies Drug Market”
- Se espera que la región Asia-Pacífico sea testigo de la mayor tasa de crecimiento en el mercado de medicamentos para las miopatías centronucleares (CNM), impulsada por los rápidos avances en la infraestructura de atención médica, la creciente conciencia sobre las enfermedades neuromusculares raras y la creciente participación en ensayos clínicos.
- Países como China, India y Japón están surgiendo como mercados clave debido al creciente reconocimiento del CNM y otros trastornos genéticos raros, junto con el enfoque creciente en la detección genética y los tratamientos personalizados.
- Japón, con su avanzada tecnología médica y su sólido sistema de salud, sigue siendo un mercado crucial para el desarrollo e investigación de fármacos para la NMC. El país continúa liderando la adopción de terapias de vanguardia y enfoques terapéuticos innovadores para enfermedades raras como la NMC.
- China e India, con sus grandes poblaciones y la creciente incidencia de trastornos genéticos, están experimentando un aumento de la inversión pública y privada en el desarrollo de fármacos para enfermedades raras. La creciente presencia de compañías farmacéuticas globales y las mejoras en el acceso a la atención médica contribuyen aún más al crecimiento del mercado.
Cuota de mercado de fármacos para las miopatías centronucleares
El panorama competitivo del mercado ofrece detalles por competidor. Se incluye información general de la empresa, sus estados financieros, ingresos generados, potencial de mercado, inversión en investigación y desarrollo, nuevas iniciativas de mercado, presencia global, plantas de producción, capacidad de producción, fortalezas y debilidades de la empresa, lanzamiento de productos, alcance y variedad de productos, y dominio de las aplicaciones. Los datos anteriores se refieren únicamente al enfoque de mercado de las empresas.
Los principales líderes del mercado que operan en el mercado son:
- Audentes Therapeutics (Estados Unidos)
- Dynacure (Francia)
- Astellas Pharma Inc. (Japón)
- Valerion Therapeutics (Estados Unidos)
- Biogen Inc. (sede: Estados Unidos)
- Ionis Pharmaceuticals (Estados Unidos)
- Sarepta Therapeutics (Estados Unidos)
- Genethon (Francia)
Últimos avances en el mercado mundial de fármacos para las miopatías centronucleares
- En enero de 2025, Dynacure presentó los resultados del estudio de fase I/II para DYN101, su candidato a fármaco antisentido para el CNM, en la conferencia de la World Muscle Society.
- En febrero de 2025, Astellas lanzó una plataforma de registro de ensayos clínicos CNM para consolidar la participación global de pacientes en todos los centros.
- En marzo de 2025, Biogen firmó un acuerdo estratégico con Genethon para el desarrollo preclínico de la terapia génica CNM.
- En abril de 2025, Ionis Pharmaceuticals inició un ensayo global que evalúa la administración sistémica de una plataforma antisentido de próxima generación para miopatías raras.
SKU-
Obtenga acceso en línea al informe sobre la primera nube de inteligencia de mercado del mundo
- Panel de análisis de datos interactivo
- Panel de análisis de empresas para oportunidades con alto potencial de crecimiento
- Acceso de analista de investigación para personalización y consultas
- Análisis de la competencia con panel interactivo
- Últimas noticias, actualizaciones y análisis de tendencias
- Aproveche el poder del análisis de referencia para un seguimiento integral de la competencia
Metodología de investigación
La recopilación de datos y el análisis del año base se realizan utilizando módulos de recopilación de datos con muestras de gran tamaño. La etapa incluye la obtención de información de mercado o datos relacionados a través de varias fuentes y estrategias. Incluye el examen y la planificación de todos los datos adquiridos del pasado con antelación. Asimismo, abarca el examen de las inconsistencias de información observadas en diferentes fuentes de información. Los datos de mercado se analizan y estiman utilizando modelos estadísticos y coherentes de mercado. Además, el análisis de la participación de mercado y el análisis de tendencias clave son los principales factores de éxito en el informe de mercado. Para obtener más información, solicite una llamada de un analista o envíe su consulta.
La metodología de investigación clave utilizada por el equipo de investigación de DBMR es la triangulación de datos, que implica la extracción de datos, el análisis del impacto de las variables de datos en el mercado y la validación primaria (experto en la industria). Los modelos de datos incluyen cuadrícula de posicionamiento de proveedores, análisis de línea de tiempo de mercado, descripción general y guía del mercado, cuadrícula de posicionamiento de la empresa, análisis de patentes, análisis de precios, análisis de participación de mercado de la empresa, estándares de medición, análisis global versus regional y de participación de proveedores. Para obtener más información sobre la metodología de investigación, envíe una consulta para hablar con nuestros expertos de la industria.
Personalización disponible
Data Bridge Market Research es líder en investigación formativa avanzada. Nos enorgullecemos de brindar servicios a nuestros clientes existentes y nuevos con datos y análisis que coinciden y se adaptan a sus objetivos. El informe se puede personalizar para incluir análisis de tendencias de precios de marcas objetivo, comprensión del mercado de países adicionales (solicite la lista de países), datos de resultados de ensayos clínicos, revisión de literatura, análisis de mercado renovado y base de productos. El análisis de mercado de competidores objetivo se puede analizar desde análisis basados en tecnología hasta estrategias de cartera de mercado. Podemos agregar tantos competidores sobre los que necesite datos en el formato y estilo de datos que esté buscando. Nuestro equipo de analistas también puede proporcionarle datos en archivos de Excel sin procesar, tablas dinámicas (libro de datos) o puede ayudarlo a crear presentaciones a partir de los conjuntos de datos disponibles en el informe.