Global Dermatology Small Molecule Api Market
Tamaño del mercado en miles de millones de dólares
Tasa de crecimiento anual compuesta (CAGR) :
% 
 USD
3.64 Billion
USD
6.26 Billion
2024
2032
USD
3.64 Billion
USD
6.26 Billion
2024
2032
| 2025 –2032 | |
| USD 3.64 Billion | |
| USD 6.26 Billion | |
|
|
|
Global Dermatology Small Molecule API Market Segmentation, By Drug Class (Anti-Infectives, Corticosteroids, Anti-Acne, Calcineurin Inhibitors, Retinoids, and Others), Application (Acne, Psoriasis, Atopic Dermatitis, and Others), Route of Administration (Oral, Parenteral, and Topical), Distribution Channel (Hospital Pharmacies, Retail Pharmacies, and Online Pharmacies), End-Use (Hospitals, Skin Clinics, and Others) - Industry Trends and Forecast to 2032
Dermatology Small Molecule API Market Analysis
The dermatology small molecule API market is experiencing steady growth driven by the increasing incidence of skin disorders such as acne, psoriasis, eczema, and atopic dermatitis. The rising awareness about skin health, coupled with the demand for more affordable treatment options, is propelling the market forward. Small molecule drugs, particularly those targeting inflammatory pathways, are being increasingly adopted due to their cost-effectiveness and ease of use compared to biologics.
A key driver for the market is the growing preference for topical small molecule therapies over systemic treatments, particularly for conditions such as acne and psoriasis. Topical drugs such as tretinoin, a retinoid, and topical corticosteroids continue to dominate the treatment of mild to moderate skin conditions due to their efficacy and relatively lower cost. Additionally, the development of novel small molecules targeting specific molecular pathways such as JAK inhibitors for atopic dermatitis and psoriasis is creating new opportunities for growth. The continued shift towards personalized medicine, where treatments are tailored to an individual's specific skin condition and genetic profile, is further boosting demand for dermatology small molecule APIs, offering promising market prospects.
Dermatology Small Molecule API Market Size
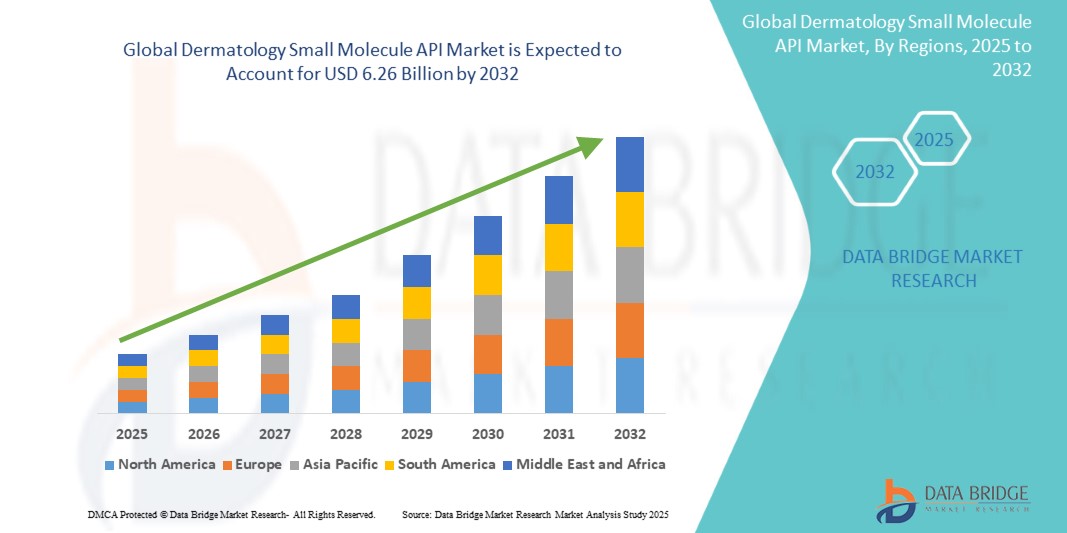
The global dermatology small molecule API market size was valued at USD 3.64 billion in 2024 and is projected to reach USD 6.26 billion by 2032, with a CAGR of 7.00% during the forecast period of 2025 to 2032. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Dermatology Small Molecule API Market Trends
“Rising Prevalence of Chronic Skin Disorders”
The main reason for the growth of the dermatology small molecule API market is the rising prevalence of chronic skin disorders such as acne, psoriasis, eczema, and atopic dermatitis, coupled with increasing patient demand for more effective and affordable treatments. As these conditions become more common worldwide, there is a growing need for both topical and systemic therapies that can manage symptoms and improve quality of life.
Small molecule drugs are gaining traction due to their cost-effectiveness, accessibility, and proven efficacy, particularly in the treatment of mild to moderate skin conditions. For instance, topical retinoids such as tretinoin and corticosteroids remain first-line treatments for acne and psoriasis, offering patients targeted relief at a lower cost compared to biologics. Additionally, the development of newer, more advanced small molecule therapies, such as JAK inhibitors for inflammatory skin diseases, is expanding the treatment options available, fueling further market growth. The shift towards personalized dermatology treatments is also driving demand, with tailored therapies increasingly sought after.
Report Scope and Dermatology Small Molecule API Market Segmentation
|
Attributes |
Dermatology Small Molecule API Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
U.S., Canada and Mexico in North America, Germany, France, U.K., Italy, Spain, Denmark, Sweden, Norway, Rest of Europe in Europe, China, Japan, India, South Korea, Australia, Thailand, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E., South Africa, Egypt, Kuwait, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America |
|
Key Market Players |
Abbott (U.S.), AbbVie Inc. (U.S.), Amgen Inc. (U.S.), Arcutis Biotherapeutics, Inc. (U.S.), BIOCAD HK Ltd (China), Bristol-Myers Squibb Company (U.S.), Can-Fite (Israel), CELLTRION INC. (Republic of Korea), Coherus BioSciences (U.S.), DermBiont, Inc. (U.S.), Galectin Therapeutics, Inc. (U.S.), Galderma (Switzerland), ILTOO Pharma (France), GSK plc. (U.K.), Johnson & Johnson Services, Inc. (U.S.), LEO Pharma A/S (Denmark), Pfizer Inc. (U.S.), Roivant Sciences Ltd. (U.K.), Sun Pharmaceutical Industries Ltd. (India), and Viatris Inc. (U.S.) |
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Dermatology Small Molecule API Market Definition
Dermatology small molecule API refers to active pharmaceutical ingredients (APIs) that are small molecules specifically developed for the treatment of dermatological conditions. These molecules are typically low molecular weight compounds that interact with specific biological targets in the body to treat skin diseases or conditions.
Dermatology Small Molecule API Market Dynamics
Drivers
- Advancements in Drug Delivery Systems
Technological advancements in drug delivery systems, including sustained-release formulations, transdermal systems, and nano-drug delivery technologies, have significantly enhanced the effectiveness and bioavailability of small molecule drugs. These innovations not only improve patient compliance but also optimize treatment outcomes, particularly for chronic dermatological conditions. For instance, Tretinoin (Retin-A), a widely used treatment for acne and aging signs, is now available in sustained-release formulations that boost its therapeutic efficacy while minimizing irritation. By enabling a controlled release of the drug, sustained-release technologies provide consistent therapeutic effects over time, enhancing long-term patient outcomes. These advancements in drug delivery systems serve as a major driver for the growth of the Dermatology Small Molecule API Market.
- Cost-Effectiveness of Small Molecule Drugs
Small molecule drugs are typically more cost-effective than biologic treatments, making them a preferred choice for both patients and healthcare systems, particularly in emerging markets. Their affordability helps improve access to essential treatments, driving market growth in countries with limited healthcare budgets. For instance, in regions such as India and Brazil, topical corticosteroids such as Hydrocortisone and Betamethasone continue to be widely prescribed for treating conditions such as eczema, acne, and psoriasis, primarily due to their lower costs compared to biologic alternatives such as anti-TNF drugs. This cost-effectiveness serves as a key driver for the growth of the Dermatology Small Molecule API Market, as it enables broader patient access and adoption, particularly in developing regions, fostering the market's expansion.
Opportunities
- Rise in Dermatological Conditions
The increasing prevalence of common skin conditions such as acne, eczema, psoriasis, and rosacea are driving the demand for small molecule treatments. As the global population seeks more effective and long-term solutions for these chronic dermatological issues, the need for small molecule APIs in dermatology continues to grow. For instance, acne vulgaris is estimated to affect 9.4% of the global population (source: Global Burden of Disease Study, 2019), representing a substantial patient base. This growing demand for treatments such as tretinoin and benzoyl peroxide highlights a clear market opportunity for small molecule drugs in dermatology. With the rising burden of skin diseases worldwide, the dermatology small molecule API market is positioned for significant expansion, creating numerous opportunities for pharmaceutical companies to develop and deliver effective solutions to a large and diverse patient population.
- Innovations in Drug Formulations
Advancements in drug delivery technologies, including sustained-release formulations, nano-drug delivery systems, and transdermal patches, are creating new opportunities for small molecule drugs in dermatology. These innovations enhance the efficacy of treatments, improve patient compliance, and minimize side effects. For instance, Tretinoin (Retin-A), a well-established small molecule used for acne treatment, is now offered in sustained-release formulations that enhance its therapeutic effectiveness while reducing skin irritation. These improvements align with the growing demand for more efficient, patient-friendly, and less irritating treatments. As drug delivery technologies continue to evolve, the dermatology small molecule API market is poised to benefit, offering new opportunities for pharmaceutical companies to address patient needs for more convenient, effective, and safer treatments.
Restraints/Challenges
- Regulatory and Approval Challenges
The dermatology small molecule API market faces regulatory hurdles that can delay product approvals, particularly for new formulations or indications. Regulatory agencies such as the FDA and EMA impose stringent requirements for clinical trials, efficacy, safety, and manufacturing standards, which can increase development timelines and costs. For instance, obtaining approval for topical drugs may require extensive clinical data to demonstrate efficacy in treating specific dermatological conditions such as acne, psoriasis, or eczema. For instance, Benzoyl Peroxide, a widely used topical treatment for acne, was initially subject to rigorous approval processes therefore, regulatory complexities are a significant restraint on the market,
- Competition from Biologic Therapies
皮膚科の低分子 API 市場は、中等度から重度の乾癬やアトピー性皮膚炎などの重度または治療抵抗性の症状に優れた有効性を示す生物学的製剤との厳しい競争に直面しています。TNF 阻害剤やモノクローナル抗体などの生物学的療法は、より的を絞った強力な効果を提供することが多いため、コストが高いにもかかわらず、複雑な皮膚疾患の治療に好まれています。たとえば、モノクローナル抗体のデュピルマブ (デュピクセント) は、中等度から重度のアトピー性皮膚炎 (AD) の治療に優れた有効性が実証されており、慢性疾患の管理における長期的な利点から、低分子治療よりも使用されることが増えています。副作用の少ない標的治療を提供する生物学的療法の好まれつつある傾向は、皮膚科の低分子 API 市場にとって大きな課題となっています。
この市場レポートでは、最近の新しい開発、貿易規制、輸出入分析、生産分析、バリュー チェーンの最適化、市場シェア、国内および現地の市場プレーヤーの影響、新たな収益源の観点から見た機会の分析、市場規制の変更、戦略的市場成長分析、市場規模、カテゴリ市場の成長、アプリケーションのニッチと優位性、製品の承認、製品の発売、地理的拡大、市場における技術革新などの詳細が提供されます。市場に関する詳細情報を取得するには、アナリスト ブリーフについて Data Bridge Market Research にお問い合わせください。当社のチームが、情報に基づいた市場決定を行い、市場の成長を実現できるようお手伝いします。
皮膚科用低分子API市場の範囲
市場は、薬物クラス、用途、投与経路、流通チャネル、最終用途に基づいてセグメント化されています。これらのセグメントの成長は、業界におけるわずかな成長セグメントの分析に役立ち、ユーザーに貴重な市場概要と市場洞察を提供し、コア市場アプリケーションを特定するための戦略的決定を下すのに役立ちます。
薬物クラス
- 抗感染薬
- コルチコステロイド
- ニキビ予防
- カルシニューリン阻害剤
- レチノイド
- その他
応用
- ニキビ
- 乾癬
- アトピー性皮膚炎
- その他
投与経路
- オーラル
- 非経口
- 話題
流通チャネル
- 病院薬局
- 小売薬局
- オンライン薬局
最終用途
- 病院
- 皮膚科クリニック
- その他
皮膚科用小分子API市場の地域分析
市場は分析され、市場規模の洞察と傾向は、上記のように国、薬物クラス、用途、投与経路、流通チャネル、および最終用途別に提供されます。
市場レポートでカバーされている国は、北米では米国、カナダ、メキシコ、ヨーロッパではドイツ、フランス、イギリス、イタリア、スペイン、デンマーク、スウェーデン、ノルウェー、その他のヨーロッパ、アジア太平洋地域 (APAC) では中国、日本、インド、韓国、オーストラリア、タイ、その他のアジア太平洋地域 (APAC)、中東およびアフリカ (MEA) の一部としてサウジアラビア、UAE、南アフリカ、エジプト、クウェート、その他の中東およびアフリカ (MEA)、南米の一部としてブラジル、アルゼンチン、その他の南米です。
北米は、医療費の増加、皮膚疾患に対する意識の高まり、大手皮膚科製薬会社の堅調な存在などの要因により、大きな市場シェアを維持すると予想されています。皮膚疾患の発生率の増加は、この地域の市場成長を推進すると予想される重要な要因です。たとえば、2023年3月に発行された世界アレルギー機構ジャーナルのレポートでは、米国におけるアトピー性皮膚炎の有病率は4.9%から10.2%の範囲であると強調されています。この皮膚疾患の有病率の高さは、国内の皮膚科治療薬の需要を大幅に促進すると予想されます。
アジア太平洋地域は、予測期間を通じて最も高い成長率を達成すると予測されています。この地域は、皮膚疾患の罹患率の増加、人口の高齢化、医療費の上昇などの要因により、皮膚科業界の主要な商業拠点として浮上しています。さらに、さまざまな政府の取り組みや協力により、皮膚科の問題への関心を高めるための取り組みが積極的に行われており、この地域の市場潜在力の拡大に貢献しています。
レポートの国別セクションでは、市場の現在および将来の動向に影響を与える国内市場における個別の市場影響要因と規制の変更も提供しています。下流および上流のバリュー チェーン分析、技術動向、ポーターの 5 つの力の分析、ケース スタディなどのデータ ポイントは、各国の市場シナリオを予測するために使用される指標の一部です。また、国別データの予測分析を提供する際には、グローバル ブランドの存在と可用性、および地元および国内ブランドとの競争が激しいか少ないために直面する課題、国内関税と貿易ルートの影響も考慮されます。
皮膚科用低分子API市場シェア
市場競争環境では、競合他社ごとの詳細が提供されます。詳細には、会社概要、会社の財務状況、収益、市場の可能性、研究開発への投資、新しい市場への取り組み、世界的なプレゼンス、生産拠点と施設、生産能力、会社の強みと弱み、製品の発売、製品の幅と広さ、アプリケーションの優位性などが含まれます。提供される上記のデータ ポイントは、市場に関連する会社の焦点にのみ関連しています。
市場で活動する皮膚科用小分子 API の市場リーダーは次のとおりです。
- アボット(米国)
- アッヴィ社(米国)
- アムジェン社(米国)
- アルクティス・バイオセラピューティクス社(米国)
- BIOCAD HK Ltd (中国)
- ブリストル・マイヤーズスクイブ社(米国)
- カンフィット(イスラエル)
- CELLTRION INC.(韓国)
- コヒーラス・バイオサイエンス(米国)
- ダームバイオント社(米国)
- ガレクチン・セラピューティクス社(米国)
- ガルデルマ(スイス)
- ILTOO Pharma(フランス)
- GSK plc. (英国)
- ジョンソン・エンド・ジョンソン・サービス社(米国)
- LEO Pharma A/S(デンマーク)
- ファイザー社(米国)
- ロイヴァント・サイエンシズ社(英国)
- サン・ファーマシューティカル・インダストリーズ(インド)
- ビアトリス社(米国)
Latest Developments in Dermatology Small Molecule API Market
- In October 2024, LEO Pharma released the financial highlights from first nine months of 2024, The company delivered revenue growth of 11% in constant exchange rates (CER). The dermatology portfolio saw revenue growth of 13%. It also included several key events around the globe such as the European Commission’s approval of Anzupgo (delgocitinib) cream for adults with moderate to severe chronic hand eczema (CHE)
- In October 2024, Organon announced the successful completion of its acquisition of Dermavant Sciences Ltd. from Roivant. Dermavant is a company dedicated to developing and commercializing innovative therapeutic solutions in immuno-dermatology. It included acquisition of VTAMA (tapinarof) cream, 1%, which is a novel nonbiologic, non-steroidal topical therapy approved by the U.S. Food and Drug Administration (FDA) for treatment of mild, moderate, and severe plaque psoriasis
- In August 2024, DermBiont, announced positive initial data from its ongoing open label, multi-center Phase 2a study for the treatment of basal cell carcinoma (BCC). The results showed that lesions which were treated with SM-020 1% gel BID (twice daily) for 28 days, with the primary endpoint based on percent change from baseline in greatest tumor diameter at week 6, showed a prompt (within days of starting treatment) and robust clinical response to the application of SM-020 1% gel, clearly demonstrating the disease modifying potential of SM-020
- In May 2024, Johnson & Johnson announced that it had reached a definitive agreement to acquire Proteologix, Inc., a privately-held biotechnology firm specializing in bispecific antibodies for immune-related diseases, for $850 million in cash, with the potential for additional milestone payments. Proteologix’s pipeline includes PX128, a bispecific antibody targeting IL-13 and TSLP, which is poised to enter Phase 1 development for the treatment of moderate to severe atopic dermatitis (AD) and moderate to severe asthma
- In March 2024, Amgen announced new, 52-week results from the Phase 3 SPROUT study examining the use of Otezla (apremilast) in children and adolescents aged 6 to 17 years with moderate to severe plaque psoriasis. Continued Otezla use resulted in sustained improvements in psoriasis severity and skin involvement in pa tients for up to one year
SKU-
Obtenga acceso en línea al informe sobre la primera nube de inteligencia de mercado del mundo
- Panel de análisis de datos interactivo
- Panel de análisis de empresas para oportunidades con alto potencial de crecimiento
- Acceso de analista de investigación para personalización y consultas
- Análisis de la competencia con panel interactivo
- Últimas noticias, actualizaciones y análisis de tendencias
- Aproveche el poder del análisis de referencia para un seguimiento integral de la competencia
Metodología de investigación
La recopilación de datos y el análisis del año base se realizan utilizando módulos de recopilación de datos con muestras de gran tamaño. La etapa incluye la obtención de información de mercado o datos relacionados a través de varias fuentes y estrategias. Incluye el examen y la planificación de todos los datos adquiridos del pasado con antelación. Asimismo, abarca el examen de las inconsistencias de información observadas en diferentes fuentes de información. Los datos de mercado se analizan y estiman utilizando modelos estadísticos y coherentes de mercado. Además, el análisis de la participación de mercado y el análisis de tendencias clave son los principales factores de éxito en el informe de mercado. Para obtener más información, solicite una llamada de un analista o envíe su consulta.
La metodología de investigación clave utilizada por el equipo de investigación de DBMR es la triangulación de datos, que implica la extracción de datos, el análisis del impacto de las variables de datos en el mercado y la validación primaria (experto en la industria). Los modelos de datos incluyen cuadrícula de posicionamiento de proveedores, análisis de línea de tiempo de mercado, descripción general y guía del mercado, cuadrícula de posicionamiento de la empresa, análisis de patentes, análisis de precios, análisis de participación de mercado de la empresa, estándares de medición, análisis global versus regional y de participación de proveedores. Para obtener más información sobre la metodología de investigación, envíe una consulta para hablar con nuestros expertos de la industria.
Personalización disponible
Data Bridge Market Research es líder en investigación formativa avanzada. Nos enorgullecemos de brindar servicios a nuestros clientes existentes y nuevos con datos y análisis que coinciden y se adaptan a sus objetivos. El informe se puede personalizar para incluir análisis de tendencias de precios de marcas objetivo, comprensión del mercado de países adicionales (solicite la lista de países), datos de resultados de ensayos clínicos, revisión de literatura, análisis de mercado renovado y base de productos. El análisis de mercado de competidores objetivo se puede analizar desde análisis basados en tecnología hasta estrategias de cartera de mercado. Podemos agregar tantos competidores sobre los que necesite datos en el formato y estilo de datos que esté buscando. Nuestro equipo de analistas también puede proporcionarle datos en archivos de Excel sin procesar, tablas dinámicas (libro de datos) o puede ayudarlo a crear presentaciones a partir de los conjuntos de datos disponibles en el informe.