Global Intra Uterine Contraceptive Devices Market
Tamaño del mercado en miles de millones de dólares
Tasa de crecimiento anual compuesta (CAGR) :
% 
 USD
4.69 Billion
USD
6.77 Billion
2024
2032
USD
4.69 Billion
USD
6.77 Billion
2024
2032
| 2025 –2032 | |
| USD 4.69 Billion | |
| USD 6.77 Billion | |
|
|
|
|
Global Intra-Uterine Contraceptive Devices Market Segmentation, By Type (Hormonal Intrauterine Device and Copper Intrauterine Device), Product Type (Mirena, Skyla, Paragard, Essure, Levosert, and Others), End User (Hospitals, Gynaecology Clinics, Community Health Care Centers, and Others) - Industry Trends and Forecast to 2032
Intra-Uterine Contraceptive Devices Market Size
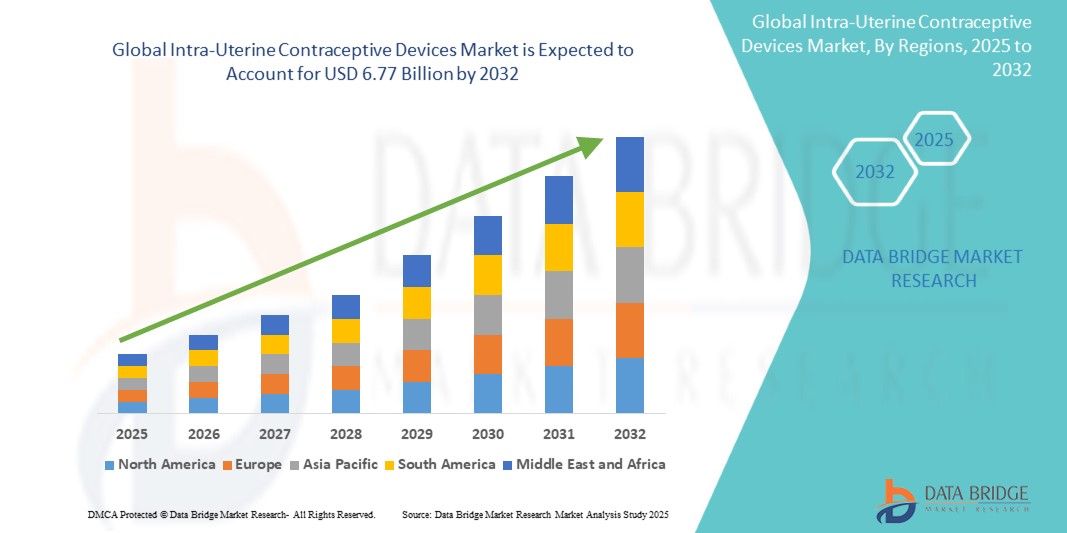
- The global intra-uterine contraceptive devices market size was valued atUSD 4.69 billion in 2024and is expected to reachUSD 6.77 billion by 2032, at aCAGR of 4.70%during the forecast period
- This growth is driven by factors such as the aging population, increasing prevalence of eye diseases, and advancements in ophthalmic technology
Intra-Uterine Contraceptive Devices Market Analysis
- Intrauterine devices (IUDs) are defined as copper birth control or small T-shaped plastic devices that are inserted into the uterus. These devices are known to prevent pregnancy as it stops sperm from reaching and fertilizing the eggs
- These devices are being highly deployed as they are considered safe, long-acting, effective and eliminating the need for other contraceptives
- Asia-Pacific is expected to dominate the intra-uterine contraceptive devices market with 34.5% due to rapidly growing population, increased demand for effective contraception, government initiatives promoting family planning
- North America is expected to be the fastest growing region in the intra-uterine contraceptive devices market during the forecast period due to increased adoption of IUDs
- Hormonal intrauterine device segment is expected to dominate the market with a market share of 72.44% due its high efficacy in preventing pregnancy, additional benefits such as reduced menstrual bleeding, improved management of menstrual symptoms and increasing awareness among users about these advantages
Report Scope and Intra-Uterine Contraceptive Devices Market Segmentation
|
Attributes |
Intra-Uterine Contraceptive Devices Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Intra-Uterine Contraceptive Devices Market Trends
“Increasing Preference for Long-Acting Reversible Contraception (LARC)”
- There is a notable global trend towards the adoption of long-acting reversible contraception methods, particularly IUDs, due to their high efficacy and convenience
- Government initiatives, such as the Affordable Care Act in the U.S., mandate coverage of contraceptives, including IUDs, without co-payments, facilitating broader access
- Medical professionals increasingly recommend IUDs for their effectiveness and minimal maintenance, contributing to their growing popularity
- Educational programs have enhanced understanding of IUD benefits, leading to informed decision-making among women
Intra-Uterine Contraceptive Devices Market Dynamics
Driver
“Government Initiatives and Healthcare Programs”
- The ACA mandates that insurance plans cover all FDA-approved contraceptive methods, including IUDs, without cost-sharing
- International initiatives aim to reduce unintended pregnancies by promoting the use of IUDs among women of reproductive age
- Programs to train healthcare professionals in IUD insertion techniques improve service delivery and patient outcomes
- Government-led campaigns raise awareness about the benefits and availability of IUDs, encouraging their use
Opportunity
“Expansion into Emerging Markets”
- Countries in Asia, Africa, and Latin America are increasing healthcare spending, improving access to contraceptive methods such as IUDs
- Initiatives such as India's 'Mission Parivar Vikas' aim to expand access to family planning services, including IUDs
- Changing societal norms are leading to greater acceptance of modern contraceptive methods among women in developing regions
- Collaborations with non-governmental organizations can enhance outreach and education efforts, increasing IUD adoption rates
- Developing culturally sensitive educational materials can address misconceptions and promote informed choices regarding IUDs
Restraint/Challenge
“Side Effects and Procedural Barriers”
- Users may experience heavy menstrual bleeding and painful cramps, leading to discontinuation of IUD use
- The insertion procedure can be uncomfortable and may require skilled healthcare providers, limiting accessibility
- In some regions, cultural and religious beliefs discourage the use of IUDs, affecting adoption rates
- Inconsistent insurance coverage for IUDs can create financial barriers for potential users
- Lack of information about IUDs in certain populations can lead to misconceptions and hesitancy in adoption
Intra-Uterine Contraceptive Devices Market Scope
The market is segmented on the basis of type, product type, and end user.
|
Segmentation |
Sub-Segmentation |
|
By Type |
|
|
By Product Type |
|
|
By End User |
|
In 2025, the hormonal intrauterine device is projected to dominate the market with a largest share in type segment
The hormonal intrauterine device segment is expected to dominate the Intra-Uterine Contraceptive Devices market with the largest share of 72.44% in 2025 due to its high efficacy in preventing pregnancy, additional benefits such as reduced menstrual bleeding, improved management of menstrual symptoms and increasing awareness among users about these advantages.
The hospital is expected to account for the largest share during the forecast period in end user market
In 2025, the hospitals segment is expected to dominate the market with the largest market share of 54.99% due to increased adoption of IUDs due to their high efficacy and long-term contraceptive benefits, coupled with hospitals' ability to provide comprehensive family planning services, including counselling and insertion.
Intra-Uterine Contraceptive Devices Market Regional Analysis
“Asia-Pacific Holds the Largest Share in the Intra-Uterine Contraceptive Devices Market”
- Asia-Pacific held the largest share of the global IUD market in 2023, accounting for 34.5% of the total market
- China and India are significant contributors to this dominance
- Programs such as India's 'Mission Parivar Vikas' aim to improve access to contraceptives, enhancing IUD adoption rates
- Rapid urbanization and expanding healthcare services in countries such as India and China facilitate broader access to IUDs
- Hormonal IUDs dominate the market in this region, with a significant preference for long-acting reversible contraceptives
“North America is Projected to Register the Highest CAGR in the Intra-Uterine Contraceptive Devices Market”
- The U.S.is witnessing a surge in demand for effective contraceptive methods, including IUDs, due to changing societal norms and increased awareness
- Governments in the region are prioritizing reproductive health, with policies aimed at improving access to family planning services and modern contraceptive methods
- Continued urbanization and improvements in healthcare infrastructure are enhancing access to IUDs, particularly in urban areas
- There is a growing acceptance of modern contraceptive methods among women in Latin America, leading to increased adoption of IUDs
- Improving economic conditions are contributing to greater purchasing power among consumers, facilitating access to IUDs
Intra-Uterine Contraceptive Devices Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- Teva Pharmaceutical Industries Ltd. (Israel)
- Bayer(Germany)
- EUROGINE(Spain)
- CooperSurgical Inc. (U.S.)
- Pfizer Inc. (U.S.)
- HLL Lifecare Limited (India)
- OCON Medical Ltd. (Israel)
- Prosan International BV (Netherlands)
- Melbea Innovations (Hungary)
- Allergan, Merck & Co., Inc. (U.S.)
- DKT International (U.S.)
- Medisafe Distribution Inc. (Canada)
- Medicines360 (U.S.)
- Pregna International Limited (India)
- Egemen International (Turkey)
Latest Developments in Global Intra-Uterine Contraceptive Devices Market
- In July 2024, Israeli women’s health companyOCONTherapeutics raised US$10 million to expand its health solutions, including painless and effective IUDs and treatment for endometriosis. The company has raised a total of USD 40 million to date, including the most recent round of funding
- In January 2024,Medicines360, a global nonprofit in women's health, and DKT WomanCare, a global distributor of contraceptive products, partnered to expand access to AVIBELA. AVIBELA is a cost-effective, high-quality hormonal IUD with over 99% effectiveness. This collaboration aims to increase awareness, accessibility, and usage of this highly effective contraceptive option
- In November 2023, pharmaceutical giantBayerannounced a development and license agreement with CrossBay Medical to create a single-handed inserter for intrauterine devices. The project involves integrating CrossBay’s CrossGlide technology with Bayer’s hormonal IUD portfolio
- In June 2023,Pregna International Limited, a company specializing in women's reproductive health, received an initial private equity investment of USD 16 million (INR 130 crore) from India Life Sciences Fund III (ILSF III), managed by the private equity firm InvAscent. This investment aims to fuel Pregna's growth and empower them to address the evolving needs of women globally
- In August 2022, Bayer AG received FDA approval for a supplemental new drug application (sNDA) for Mirena IUD, extending its period of use to up to eight years. This approval is intended to ensure that women have access to the contraceptive options needed at various stages of their reproductive life
- In June’2021,Sebela Pharmaceuticals Inc., collaborated with PRA Health Sciences to conduct a phase III clinical trial to assess LevoCept. The product is a long-acting reversible intrauterine system for contraceptive safety, tolerability and efficacy. The trial is going to be completed
SKU-
Obtenga acceso en línea al informe sobre la primera nube de inteligencia de mercado del mundo
- Panel de análisis de datos interactivo
- Panel de análisis de empresas para oportunidades con alto potencial de crecimiento
- Acceso de analista de investigación para personalización y consultas
- Análisis de la competencia con panel interactivo
- Últimas noticias, actualizaciones y análisis de tendencias
- Aproveche el poder del análisis de referencia para un seguimiento integral de la competencia
Metodología de investigación
La recopilación de datos y el análisis del año base se realizan utilizando módulos de recopilación de datos con muestras de gran tamaño. La etapa incluye la obtención de información de mercado o datos relacionados a través de varias fuentes y estrategias. Incluye el examen y la planificación de todos los datos adquiridos del pasado con antelación. Asimismo, abarca el examen de las inconsistencias de información observadas en diferentes fuentes de información. Los datos de mercado se analizan y estiman utilizando modelos estadísticos y coherentes de mercado. Además, el análisis de la participación de mercado y el análisis de tendencias clave son los principales factores de éxito en el informe de mercado. Para obtener más información, solicite una llamada de un analista o envíe su consulta.
La metodología de investigación clave utilizada por el equipo de investigación de DBMR es la triangulación de datos, que implica la extracción de datos, el análisis del impacto de las variables de datos en el mercado y la validación primaria (experto en la industria). Los modelos de datos incluyen cuadrícula de posicionamiento de proveedores, análisis de línea de tiempo de mercado, descripción general y guía del mercado, cuadrícula de posicionamiento de la empresa, análisis de patentes, análisis de precios, análisis de participación de mercado de la empresa, estándares de medición, análisis global versus regional y de participación de proveedores. Para obtener más información sobre la metodología de investigación, envíe una consulta para hablar con nuestros expertos de la industria.
Personalización disponible
Data Bridge Market Research es líder en investigación formativa avanzada. Nos enorgullecemos de brindar servicios a nuestros clientes existentes y nuevos con datos y análisis que coinciden y se adaptan a sus objetivos. El informe se puede personalizar para incluir análisis de tendencias de precios de marcas objetivo, comprensión del mercado de países adicionales (solicite la lista de países), datos de resultados de ensayos clínicos, revisión de literatura, análisis de mercado renovado y base de productos. El análisis de mercado de competidores objetivo se puede analizar desde análisis basados en tecnología hasta estrategias de cartera de mercado. Podemos agregar tantos competidores sobre los que necesite datos en el formato y estilo de datos que esté buscando. Nuestro equipo de analistas también puede proporcionarle datos en archivos de Excel sin procesar, tablas dinámicas (libro de datos) o puede ayudarlo a crear presentaciones a partir de los conjuntos de datos disponibles en el informe.