North America Antiviral Drugs Market, By Indication (Influenza, Human Immunodeficiency Virus (HIV), Hepatitis C Virus (HCV), Respiratory Syncytial Virus, Herpes Simplex Virus, Human Cytomegalovirus (HCMV), Varicella-Zoster Virus (VZV), Hepatitis B Virus (HBV), Coronavirus Infection, and Others), Patient Type (Child, Adult, and Geriatric), Products (Oral, Topical, and Parenteral), Drug Type (Generic and Branded), End User (Hospitals, Clinics, Home Healthcare, Specialty Centers, Ambulatory Centers, and Others), Distribution Channel (Hospital Pharmacy, Online Pharmacy, and Retail Pharmacy) - Industry Trends and Forecast to 2030.
North America Antiviral Drugs Market Analysis and Insights
The increasing awareness about viral infections North America has enhanced the demand for the market. The rising healthcare expenditure for better health services also contributes to the market's growth. The major market players focus on various service launches and approvals during this crucial period. In addition, the increase in improved advancement of drug development techniques also contributes to the rising demand for antiviral drugs.
The North America antiviral drugs market is expected to grow in the forecast year due to the rise in market players and the availability of advanced services. Along with this, manufacturers are engaged in the developmental activity for launching novel services in the market. The increasing development in the field of drug development is further boosting the market growth. However, difficulties such as the lack of standardized protocols and the lack of skilled professionals might hamper the growth of the North America antiviral drugs market in the forecast period.
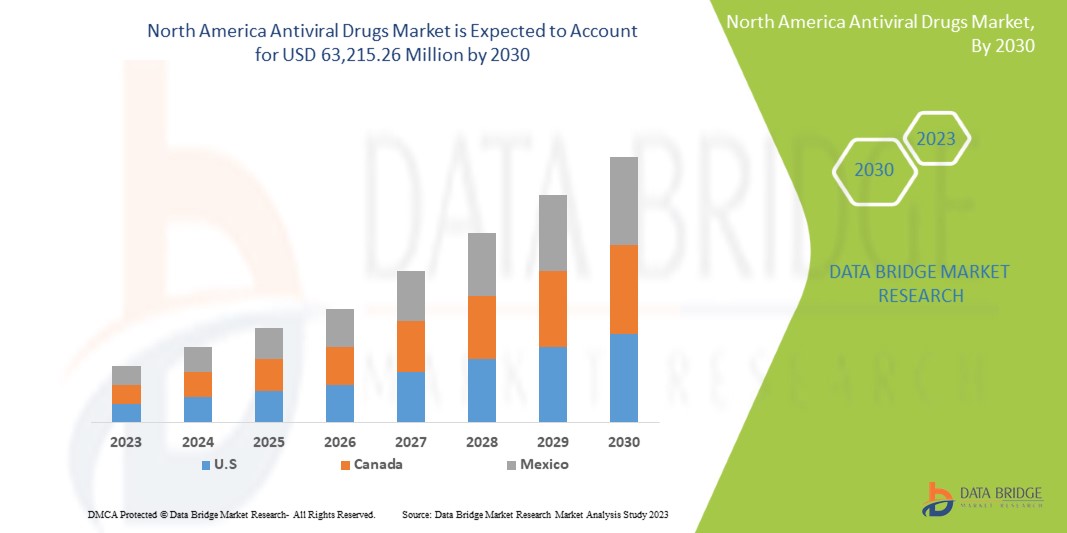
Increasing healthcare expenditure on advancement and drug development is expected to give opportunities to the market. However, the increasing use of alternative treatments may challenge market growth. Data Bridge Market Research analyzes that the North America antiviral drugs market is expected to reach the value of USD 63,215.26 million by 2030, with a CAGR of 5.5% during the forecast period.
|
Report Metric |
Details |
|
Forecast Period |
2023 to 2030 |
|
Base Year |
2022 |
|
Historic Years |
2021 (Customizable to 2015-2020) |
|
Quantitative Units |
Revenue in Million, Volumes in Units, and Pricing in USD |
|
Segments Covered |
Indicación (influenza, virus de inmunodeficiencia humana (VIH), virus de la hepatitis C (VHC), virus respiratorio sincitial, virus del herpes simple, citomegalovirus humano (CMVH), virus de la varicela-zóster (VZV), virus de la hepatitis B (VHB), infección por coronavirus y otros), tipo de paciente (niño, adulto y geriátrico), productos (oral, tópico y parenteral), tipo de medicamento (genérico y de marca), usuario final (hospitales, clínicas, atención médica domiciliaria , centros especializados, centros ambulatorios y otros), canal de distribución (farmacia hospitalaria, farmacia en línea y farmacia minorista) |
|
Países cubiertos |
Estados Unidos, Canadá y México |
|
Actores del mercado cubiertos |
Gilead Sciences, Inc., F. Hoffmann-La Roche Ltd, GLAXOSMITHKLINE PLC, Abbvie, Merck & Co., Inc., Johnson & Johnson Services, Inc., Bristol-Myers Squibb Company, Cipla Inc., Aurobindo Pharma, Dr. Reddy's Laboratories Ltd., Zydus Pharmaceuticals, Inc., Mylan Pharmaceuticals ULC, Teva Pharmaceuticals USA, Inc., EMERGENT, Sun Pharmaceutical Industries Ltd., Avet Pharmaceuticals Inc., Pfizer Inc., SIGA Technologies, NAVINTA LLC., Macleods Pharmaceuticals Ltd., BioCryst Pharmaceuticals, Inc. y Hetero, entre otros. |
Definición del mercado de medicamentos antivirales de América del Norte
Los medicamentos antivirales son medicamentos que se utilizan para tratar infecciones virales inhibiendo la replicación de los virus dentro de las células huésped. Estos medicamentos se dirigen a virus o tipos de virus específicos y funcionan impidiendo que el virus entre en la célula huésped o bloqueando enzimas o proteínas clave necesarias para la replicación viral. A diferencia de los antibióticos , que se utilizan para tratar infecciones bacterianas, los medicamentos antivirales son generalmente menos eficaces, ya que los virus tienen una estructura mucho más simple y dependen de las células huésped para replicarse. Sin embargo, aún pueden ser útiles para tratar algunas infecciones virales, como la gripe, el herpes y el VIH.
Dinámica del mercado de medicamentos antivirales en América del Norte
En esta sección se aborda la comprensión de los factores impulsores, las oportunidades, las limitaciones y los desafíos del mercado. Todo esto se analiza en detalle a continuación:
Conductores
- Aumento de la prevalencia de infecciones virales
Las infecciones virales han aumentado de manera constante en todo el mundo durante las últimas décadas. Un virus que ingresa al cuerpo, explota sus células para replicarse y se propaga provoca una infección viral. Las infecciones virales pueden provocar diversos síntomas, desde leves a graves y, en algunos casos, incluso mortales. La globalización es una de las principales causas del aumento de las infecciones virales. Las personas viajan y se comunican entre sí a través de las fronteras, lo que hace que el mundo esté más conectado que nunca. La transmisión viral de una zona a otra se ha acelerado debido a esta mayor conectividad.
Por lo tanto, la creciente prevalencia de infecciones virales es un problema complejo al que contribuyen muchos factores. La globalización, la densidad de población, el cambio climático y la resistencia a los antibióticos influyen en la propagación de los virus, por lo que se espera que impulse el crecimiento del mercado.
- Avances en el desarrollo de nuevos fármacos antivirales
A los pacientes con infecciones virales se les recetan tratamientos antivirales. La creación de nuevos medicamentos antivirales ha avanzado enormemente a lo largo del tiempo. Estos avances han reducido la carga de la enfermedad, han mejorado el tratamiento de las infecciones virales y han salvado vidas. Se ha producido un gran avance en el campo de los nuevos medicamentos antivirales.
Por lo tanto, los avances en el desarrollo de nuevos fármacos antivirales han mejorado el tratamiento de las infecciones virales, han reducido la carga de la enfermedad y se espera que impulsen el crecimiento del mercado.
Restricción
- Alto costo de los medicamentos antivirales
El alto costo de los medicamentos antivirales puede tener consecuencias importantes para los pacientes y los sistemas de atención de la salud. Los pacientes que no pueden costearlos pueden prescindir del tratamiento o recurrir a tratamientos inferiores, lo que conduce a peores resultados en materia de salud. Además, el alto costo de los medicamentos antivirales puede afectar los presupuestos de atención de la salud, en particular en países con recursos limitados.
Por lo tanto, se espera que el alto costo de los medicamentos antivirales restrinja el mercado de medicamentos antivirales en América del Norte.
Oportunidad
-
Nuevos sistemas de administración de fármacos en auge
La investigación sobre medicamentos antivirales ha puesto el énfasis en la creación de nuevos mecanismos de administración de fármacos. En comparación con las técnicas de administración de fármacos convencionales, los nuevos sistemas de administración presentan varias ventajas, como una mayor biodisponibilidad, una administración personalizada del fármaco y menos efectos adversos.
Por lo tanto, el desarrollo de nuevos sistemas de administración de fármacos es un área importante de investigación en el campo de los fármacos antivirales. Los sistemas de administración de fármacos basados en nanopartículas, hidrogeles, dendrímeros, microagujas y péptidos que penetran en las células son algunos de los sistemas de administración de fármacos prometedores que se han investigado para los fármacos antivirales. Estos sistemas de administración ofrecen varias ventajas con respecto a los métodos tradicionales de administración de fármacos y tienen el potencial de mejorar la eficacia y la seguridad de los fármacos antivirales, por lo que se espera que creen una oportunidad en el crecimiento del mercado.
Desafío
- Caducidad de las patentes de los medicamentos antivirales
El proceso de expiración de una patente hace que el desarrollador o titular original de la patente pierda su derecho exclusivo a producir y comercializar un medicamento específico. La expiración de la patente de los medicamentos antivirales puede afectar significativamente al negocio farmacéutico, ya que puede generar competencia de los productores de medicamentos genéricos.
Las patentes de los medicamentos antivirales expiran al final del período en el que su creador tiene el derecho exclusivo de fabricar y comercializar el medicamento. Una vez que expira la patente de un medicamento, otros productores pueden crear y comercializar versiones genéricas, lo que puede generar una mayor competencia y precios más bajos para el consumidor. El VIH, la hepatitis B y C, el herpes, la gripe y otras enfermedades virales se tratan con medicamentos antivirales. Las patentes de los medicamentos antivirales expiran en diferentes momentos según el medicamento y el país. Las patentes de medicamentos se otorgan normalmente por 20 años a partir de la fecha de presentación. Otros productores tienen libertad para crear y comercializar versiones genéricas del medicamento una vez que su patente ha expirado. Debido a que el productor no tiene que gastar tanto en marketing, investigación y desarrollo y estudios clínicos, los medicamentos genéricos suelen ser más asequibles que los medicamentos de marca.
Por lo tanto, la expiración de las patentes de los medicamentos antivirales puede tener implicaciones significativas para la disponibilidad, asequibilidad y accesibilidad de estos importantes medicamentos y se espera que actúe como un desafío para el crecimiento del mercado.
Acontecimientos recientes
- En enero de 2023, Merck, conocida como MSD, anunció la finalización exitosa de la oferta pública de adquisición en efectivo, a través de una subsidiaria, de todas las acciones ordinarias en circulación de Imago Biosciences, Inc. (Nasdaq: IMGO), a un precio de compra de USD 36,00 por acción en efectivo, sin intereses y sujeto a la deducción de cualquier retención fiscal requerida. La adquisición ayudará al crecimiento de los ingresos.
- En abril de 2021, Zydus Pharmaceuticals, Inc. anunció que había recibido la aprobación de uso de emergencia restringido del Controlador General de Medicamentos de la India (DCGI) para utilizar el medicamento antiviral Virafin para el tratamiento de infecciones moderadas por COVID-19. Esto ayudará a la empresa a aumentar su presencia en América del Norte y su reputación en otras regiones del mundo.
Panorama del mercado de medicamentos antivirales en América del Norte
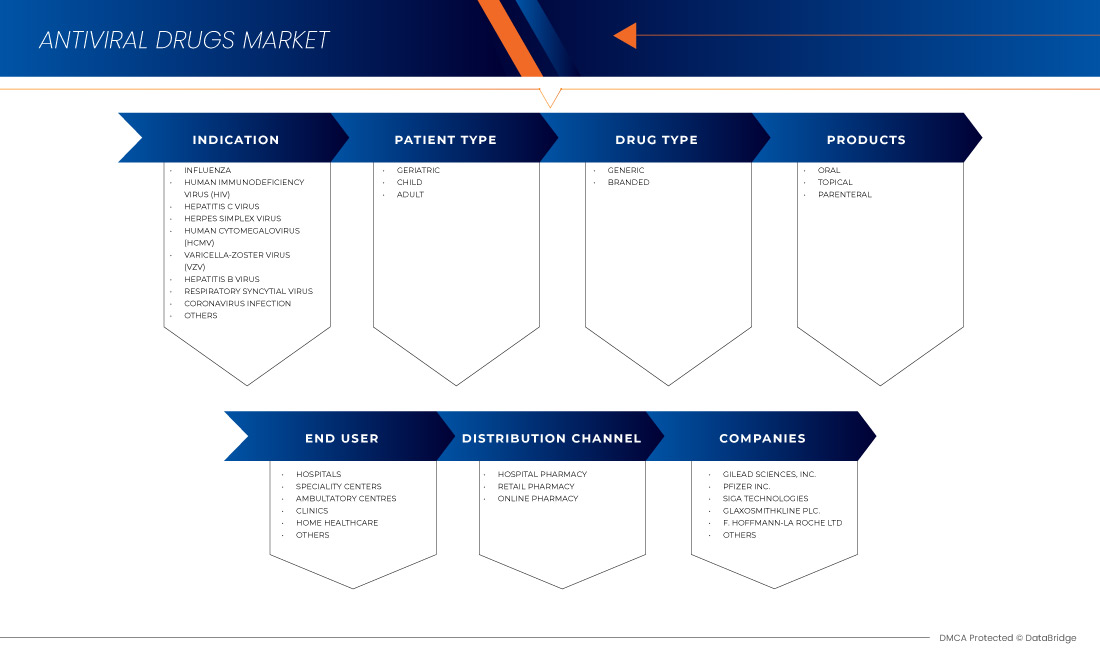
El mercado de medicamentos antivirales de América del Norte está segmentado en seis segmentos notables según la indicación, el tipo de paciente, los productos, el tipo de medicamento, el usuario final y el canal de distribución. El crecimiento entre segmentos le ayuda a analizar nichos de crecimiento y estrategias para abordar el mercado y determinar sus áreas de aplicación principales y la diferencia en sus mercados objetivo.
Indicación
- Influenza
- Virus de inmunodeficiencia humana (VIH)
- Virus de la hepatitis C
- Virus del herpes simple
- Citomegalovirus humano (HCMV)
- Virus de la varicela-zóster (VZV)
- Virus de la hepatitis B
- Virus sincitial respiratorio
- Contagio de coronavirus
- Otros
Sobre la base de la indicación, el mercado de medicamentos antivirales de América del Norte está segmentado en influenza, virus de inmunodeficiencia humana (VIH), virus de la hepatitis C (VHC), virus respiratorio sincitial, virus del herpes simple, citomegalovirus humano (HCMV), virus varicela-zóster (VZV), virus de la hepatitis B (VHB), infección por coronavirus y otros.
Tipo de paciente
- Niño
- Adulto
- Geriátrico
Según el tipo de paciente, el mercado de medicamentos antivirales de América del Norte está segmentado en niños, adultos y geriátricos.
PRODUCTOS
- Oral
- Actual
- Parenteral
Sobre la base de los productos, el mercado de medicamentos antivirales de América del Norte está segmentado en orales, tópicos y parenterales.
Tipo de droga
- Genérico
- De marca
Según el tipo de medicamento, el mercado de medicamentos antivirales de América del Norte está segmentado en genéricos y de marca.
Usuario final
- Hospital
- Clínicas
- Atención médica domiciliaria
- Centros de especialidades
- Centros ambulatorios
- Otros
Sobre la base del usuario final, el mercado de medicamentos antivirales de América del Norte está segmentado en hospitales, clínicas, atención médica domiciliaria, centros especializados, centros ambulatorios y otros.
Canal de distribución
- Farmacia hospitalaria
- Farmacia en línea
- Farmacia minorista
Sobre la base del canal de distribución, el mercado de medicamentos antivirales de América del Norte está segmentado en farmacia hospitalaria, farmacia en línea y farmacia minorista.
Análisis y perspectivas regionales del mercado de medicamentos antivirales de América del Norte
El mercado de medicamentos antivirales de América del Norte se clasifica en seis segmentos notables según la indicación, el tipo de paciente, los productos, el tipo de medicamento, el usuario final y el canal de distribución.
Los países cubiertos en este informe de mercado son Estados Unidos, Canadá y México.
En 2023, EE. UU. domina la región de América del Norte debido a la fuerte presencia de actores clave y debido a la creciente demanda de los mercados emergentes y la expansión.
La sección de países del informe también proporciona factores de impacto individuales en el mercado y cambios en la regulación en el mercado a nivel nacional que afectan las tendencias actuales y futuras del mercado. Los puntos de datos como nuevas ventas, ventas de reemplazo, demografía del país, leyes regulatorias y aranceles de importación y exportación son algunos de los principales indicadores utilizados para pronosticar el escenario del mercado para países individuales. Además, la presencia y disponibilidad de marcas de América del Norte y sus desafíos enfrentados debido a la competencia grande o escasa de las marcas locales y nacionales, y el impacto de los canales de venta se consideran al proporcionar un análisis de pronóstico de los datos del país.
Análisis del panorama competitivo y de la cuota de mercado de medicamentos antivirales en América del Norte
El panorama competitivo del mercado de medicamentos antivirales de América del Norte proporciona detalles por competidor. Los detalles incluidos son una descripción general de la empresa, las finanzas de la empresa, los ingresos generados, el potencial de mercado, la inversión en I+D, las nuevas iniciativas de mercado, los sitios e instalaciones de producción, las fortalezas y debilidades de la empresa, el lanzamiento de productos, las aprobaciones de productos, la amplitud y el alcance de los productos, el dominio de las aplicaciones y la curva de supervivencia del tipo de producto. Los puntos de datos anteriores proporcionados solo están relacionados con el enfoque de la empresa en el mercado de medicamentos antivirales de América del Norte.
Algunos de los principales actores que operan en el mercado de medicamentos antivirales de América del Norte son Gilead Sciences, Inc., F. Hoffmann-La Roche Ltd, GLAXOSMITHKLINE PLC, Abbvie, Merck & Co., Inc., Johnson & Johnson Services, Inc., Bristol-Myers Squibb Company, Cipla Inc., Aurobindo Pharma, Dr. Reddy's Laboratories Ltd., Zydus Pharmaceuticals, Inc., Mylan Pharmaceuticals ULC, Teva Pharmaceuticals USA, Inc., EMERGENT, Sun Pharmaceutical Industries Ltd., Avet Pharmaceuticals Inc., Pfizer Inc., SIGA Technologies, NAVINTA LLC., Macleods Pharmaceuticals Ltd., BioCryst Pharmaceuticals, Inc y Hetero, entre otros.
SKU-
Obtenga acceso en línea al informe sobre la primera nube de inteligencia de mercado del mundo
- Panel de análisis de datos interactivo
- Panel de análisis de empresas para oportunidades con alto potencial de crecimiento
- Acceso de analista de investigación para personalización y consultas
- Análisis de la competencia con panel interactivo
- Últimas noticias, actualizaciones y análisis de tendencias
- Aproveche el poder del análisis de referencia para un seguimiento integral de la competencia
Tabla de contenido
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF THE NORTH AMERICA ANTIVIRAL DRUGS MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 PRODUCT TYPE LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET TESTING TYPE COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PORTER’S FIVE FORCES
4.2 PESTEL ANALYSIS
5 EPIDEMIOLOGY
6 PIPELINE ANALYSIS FOR NORTH AMERICA ANTIVIRAL DRUGS MARKET
7 REGULATORY FRAMEWORK
8 MARKET OVERVIEW
8.1 DRIVERS
8.1.1 RISING PREVALENCE OF VIRAL INFECTIONS
8.1.2 ADVANCEMENTS IN NEW ANTIVIRAL DRUG DEVELOPMENT
8.1.3 GROWING DEMAND FOR COMBINATION THERAPIES
8.1.4 INCREASING GOVERNMENT FUNDING AND R&D ACTIVITIES
8.2 RESTRAINS
8.2.1 HIGH COST OF ANTIVIRAL DRUGS
8.2.2 EMERGENCE OF DRUG-RESISTANT STRAINS OF VIRUSES
8.3 OPPORTUNITIES
8.3.1 INCREASING COLLABORATION AND PARTNERSHIP AMONG KEY PLAYERS
8.3.2 RISING NOVEL DRUG DELIVERY SYSTEMS
8.3.3 DEVELOPMENT OF PERSONALIZED MEDICINES
8.4 CHALLENGES
8.4.1 PATENT EXPIRATION OF ANTIVIRAL DRUGS
8.4.2 DEMAND FOR ALTERNATIVE MEDICINES
9 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY INDICATION
9.1 OVERVIEW
9.2 INFLUENZA
9.2.1 NEURAMINIDASE INHIBITORS
9.2.1.1 OSELTAMIVIR
9.2.1.2 ZANAMIVIR
9.2.1.3 PERAMIVIR
9.2.1.4 LANINAMIVIR
9.2.2 M2 INHIBITORS
9.2.2.1 RIMANTADINE
9.2.2.2 OTHERS
9.2.3 RNA POLYMERASE INHIBITORS
9.2.3.1 FAVIPIRAVIR
9.2.3.2 BALOXAVIR MARBOXIL
9.3 HUMAN IMMUNODEFICIENCY VIRUS (HIV)
9.3.1 REVERSE TRANSCRIPTASE INHIBITORS
9.3.1.1 NUCLEOSIDE (NRTIS)
9.3.1.1.1 LAMIVUDINE
9.3.1.1.2 ABACAVIR
9.3.1.1.3 DIDANOSINE
9.3.1.1.4 OTHERS
9.3.1.2 NONNUCLEOSIDE (NNRTIS)
9.3.1.2.1 EFAVIRENZ
9.3.1.2.2 NEVIRAPINE
9.3.1.2.3 DELAVIRDINE
9.3.1.2.4 OTHERS
9.3.1.3 INTEGRASE
9.3.1.3.1 DOLUTEGRAVIR
9.3.1.3.2 ELVITEGRAVIR
9.3.1.3.3 RALTEGRAVIR
9.3.1.3.4 BICTEGRAVIR
9.3.1.4 NUCLEOTIDE
9.3.1.4.1 TENOFOVIR
9.3.1.4.2 OTHERS
9.3.1.5 INTERFERONS
9.3.1.5.1 ALPHA
9.3.1.5.2 BETA
9.3.1.5.3 GAMMA
9.3.1.6 GP41
9.3.1.6.1 ENFUVIRTIDE
9.3.1.6.2 OTHERS
9.3.2 PROTEASE
9.3.2.1 ATAZANAVIR
9.3.2.2 DARUNAVIR
9.3.2.3 LOPINAVIR
9.3.2.4 RITONAVIR
9.3.2.5 SAQUINAVIR
9.3.2.6 INDINAVIR
9.3.2.7 NELFINAVIR
9.3.2.8 TIPRANAVIR
9.3.2.9 AMPRENAVIR
9.4 HEPATITIS C VIRUS
9.4.1 NS5B POLYMERASE
9.4.1.1 SOFOSBUVIR
9.4.1.2 DASABUVIR
9.4.2 NS3/4A PROTEASE
9.4.2.1 DANOPREVIR
9.4.2.2 GLECAPREVIR
9.4.2.3 GRAZOPREVIR
9.4.2.4 PARITAPREVIR
9.4.2.5 SIMEPREVIR
9.4.3 NS5A PHOSPHOPROTEIN
9.4.3.1 LEDIPASVIR
9.4.3.2 VELPATASVIR
9.4.3.3 OMBITASVIR
9.4.3.4 ELBASVIR
9.4.3.5 DACLATASVIR
9.4.3.6 PIBRENTASVIR
9.4.4 NEURAMINIDASE
9.4.4.1 OSELTAMIVIR
9.4.4.2 ZANAMIVIR
9.4.4.3 PERAMIVIR
9.4.4.4 LANINAMIVIR
9.4.5 RNA POLYMERASE
9.4.5.1 BALOXAVIR MARBOXIL
9.4.5.2 FAVIPIRAVIR
9.4.6 MATRIX PROTEIN 2
9.4.6.1 RIMATIDINE
9.4.6.2 FAVIPIRAVIR
9.5 HERPES SIMPLEX VIRUS
9.5.1 DNA POLYMERASE UL30
9.5.1.1 ACICLOVIR
9.5.1.2 FAMCICLOVIR
9.5.1.3 VALACICLOVIR
9.5.1.4 PENCICLOVIR TRIFLURIDINE
9.5.1.5 BRIVUDINE
9.5.1.6 FOSCARNET
9.5.1.7 IDOXURIDINE
9.5.2 ENVELOPE PROTEINS
9.5.2.1 DOCOSANOL
9.5.2.2 OTHERS
9.6 HUMAN CYTOMEGALOVIRUS (HCMV)
9.6.1 GANCICLOVIR
9.6.2 VALGANCICLOVIR
9.6.3 CIDOFOVIR
9.6.4 FOSCARNET
9.6.5 FOMIVIRSEN
9.7 VARICELLA-ZOSTER VIRUS (VZV)
9.7.1 VALACICLOVIR
9.7.2 FAMCICLOVIR
9.7.3 ACICLOVIR
9.7.4 VIDARABINE
9.7.5 BRIVUDINE
9.8 HEPATITIS B VIRUS
9.8.1 ENTECAVIR
9.8.2 TENOFOVIR
9.8.3 TELBIVUDINE
9.8.4 TENOFOVIR ALAFENAMIDE
9.8.5 OTHERS
9.9 RESPIRATORY SYNCYTIAL VIRUS
9.9.1 RNA POLYMERASE
9.9.1.1 RIBAVIRIN
9.9.1.2 OTHERS
9.9.2 FUSION GLYCOPROTEIN
9.9.2.1 PALIVIZUMAB
9.9.2.2 OTHERS
9.1 CORONAVIRUS INFECTION
9.11 OTHERS
10 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE
10.1 OVERVIEW
10.2 GERIATRIC
10.2.1 MALE
10.2.2 FEMALE
10.3 CHILD
10.3.1 MALE
10.3.2 FEMALE
10.4 ADULT
10.4.1 MALE
10.4.2 FEMALE
11 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY PRODUCTS
11.1 OVERVIEW
11.2 ORAL
11.2.1 SOLID
11.2.1.1 TABLETS
11.2.1.2 CAPSULES
11.2.1.3 OTHERS
11.2.2 SEMISOLID
11.2.2.1 GELS
11.2.2.2 EMULSIONS
11.2.2.3 ELIXIRS
11.2.2.4 OTHERS
11.2.3 LIQUID
11.2.3.1 SOLUTIONS
11.2.3.2 SYRUPS
11.2.3.3 OTHERS
11.3 TOPICAL
11.3.1 SEMI-SOLID
11.3.1.1 CREAM
11.3.1.2 OINTMENT
11.3.1.3 GELS
11.3.1.4 OTHERS
11.3.2 LIQUID
11.3.2.1 SOLUTIONS
11.3.2.2 SUSPENSIONS
11.3.3 SOLID
11.3.3.1 POWDERS
11.3.3.2 SUPPOSITORIES
11.3.3.3 ENEMA
11.3.3.4 OTHERS
11.4 PARENTERAL
11.4.1 CONVENTIONAL DRUG DELIVERY FORMUALTIONS
11.4.1.1 SOLUTIONS
11.4.1.2 RECONSTITUTED/LYOPHILIZED
11.4.1.3 SUSPENSIONS
11.4.1.4 EMULSIONS
11.4.1.5 OTHERS
11.4.2 NOVEL DRUG DELIVERY FORMULATIONS
11.4.3 COLLOIDAL DISPERSIONS
11.4.4 LONG ACTING INJECTION FORMULATION
12 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY DRUG TYPE
12.1 OVERVIEW
12.2 GENERIC
12.3 BRANDED
13 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY END USER
13.1 OVERVIEW
13.2 HOSPITAL
13.3 SPECIALTY CENTERS
13.4 AMBULATORY CENTRES
13.5 CLINICS
13.6 HOME HEALTHCARE
13.7 OTHERS
14 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY DISTRIBUTION CHANNEL
14.1 OVERVIEW
14.2 HOSPITAL PHARMACY
14.3 RETAIL PHARMACY
14.4 ONLINE PHARMACY
15 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY REGION
15.1 NORTH AMERICA
15.1.1 U.S.
15.1.2 CANADA
15.1.3 MEXICO
16 NORTH AMERICA ANTIVIRAL DRUGS MARKET: COMPANY LANDSCAPE
16.1 COMPANY SHARE ANALYSIS: NORTH AMERICA
17 SWOT ANALYSIS
18 COMPANY PROFILE
18.1 GILEAD SCIENCES, INC. (2022)
18.1.1 COMPANY SNAPSHOT
18.1.2 REVENUE ANALYSIS
18.1.3 COMPANY SHARE ANALYSIS
18.1.4 PRODUCT PORTFOLIO
18.1.5 RECENT DEVELOPMENT
18.2 PFIZER INC. (2022)
18.2.1 COMPANY SNAPSHOT
18.2.2 REVENUE ANALYSIS
18.2.3 COMPANY SHARE ANALYSIS
18.2.4 PRODUCT PORTFOLIO
18.2.5 RECENT DEVELOPMENTS
18.3 SIGA TECHNOLOGIES (2022)
18.3.1 COMPANY SNAPSHOT
18.3.2 REVENUE ANALYSIS
18.3.3 COMPANY SHARE ANALYSIS
18.3.4 PRODUCT PORTFOLIO
18.3.5 RECENT DEVELOPMENT
18.4 GLAXOSMITHKLINE PLC.
18.4.1 COMPANY SNAPSHOT
18.4.2 REVENUE ANALYSIS
18.4.3 COMPANY SHARE ANALYSIS
18.4.4 PRODUCT PORTFOLIO
18.4.5 RECENT DEVELOPMENT
18.5 F. HOFFMANN-LA ROCHE LTD. (2022)
18.5.1 COMPANY SNAPSHOT
18.5.2 REVENUE ANALYSIS
18.5.3 COMPANY SHARE ANALYSIS
18.5.4 PRODUCT PORTFOLIO
18.5.5 RECENT DEVELOPMENT
18.6 ABBVIE INC.
18.6.1 COMPANY SNAPSHOT
18.6.2 REVENUE ANALYSIS
18.6.3 PRODUCT PORTFOLIO
18.6.4 RECENT DEVELOPMENT
18.7 AUROBINDO PHARMA (2022)
18.7.1 COMPANY SNAPSHOT
18.7.2 REVENUE ANALYSIS
18.7.3 PRODUCT PORTFOLIO
18.7.4 RECENT DEVELOPMENT
18.8 AVET PHARMACEUTICALS INC.
18.8.1 COMPANY SNAPSHOT
18.8.2 PRODUCT PORTFOLIO
18.8.3 RECENT DEVELOPMENT
18.9 BRISTOLL MYERS SQUIBB (2022)
18.9.1 COMPANY SNAPSHOT
18.9.2 REVENUE ANALYSIS
18.9.3 PRODUCT PORTFOLIO
18.9.4 RECENT DEVELOPMENT
18.1 BIOCRYST PHARMACEUTICALS, INC. (2022)
18.10.1 COMPANY SNAPSHOT
18.10.2 REVENUE ANALYSIS
18.10.3 PRODUCT PORTFOLIO
18.10.4 RECENT DEVELOPMENT
18.11 CIPLA INC. (2022)
18.11.1 COMPANY SNAPSHOT
18.11.2 REVENUE ANALYSIS
18.11.3 PRODUCT PORTFOLIO
18.11.4 RECENT DEVELOPMENT
18.12 DR. REDDY’S LABORATORIES LTD. (2022)
18.12.1 COMPANY SNAPSHOT
18.12.2 REVENUE ANALYSIS
18.12.3 PRODUCT PORTFOLIO
18.12.4 RECENT DEVELOPMENTS
18.13 EMERGENT (2022)
18.13.1 COMPANY SNAPSHOT
18.13.2 REVENUE ANALYSIS
18.13.3 PRODUCT PORTFOLIO
18.13.4 RECENT DEVELOPMENT
18.14 HETERO.
18.14.1 COMPANY SNAPSHOT
18.14.2 PRODUCT PORTFOLIO
18.14.3 RECENT DEVELOPMENT
18.15 JOHNSON & JOHNSON PRIVATE LIMITED (2022)
18.15.1 COMPANY SNAPSHOT
18.15.2 REVENUE ANALYSIS
18.15.3 PRODUCT PORTFOLIO
18.15.4 RECENT DEVELOPMENT
18.16 MACLEODS PHARMACEUTICALS LTD.
18.16.1 COMPANY SNAPSHOT
18.16.2 PRODUCT PORTFOLIO
18.16.3 RECENT DEVELOPMENT
18.17 MERCK & CO., INC, (2022)
18.17.1 COMPANY SNAPSHOT
18.17.2 REVENUE ANALYSIS
18.17.3 PRODUCT PORTFOLIO
18.17.4 RECENT DEVELOPMENT
18.18 MYLAN N.V (SUBSIDIARY OF VIATRIS) (2022)
18.18.1 COMPANY SNAPSHOT
18.18.2 REVENUE ANALYSIS
18.18.3 PRODUCT PORTFOLIO
18.18.4 RECENT DEVELOPMENT
18.19 NAVINTA LLC.
18.19.1 COMPANY SNAPSHOT
18.19.2 PRODUCT PORTFOLIO
18.19.3 RECENT DEVELOPMENT
18.2 SUN PHARMACEUTICAL INDUSTRIES LTD. (2022)
18.20.1 COMPANY SNAPSHOT
18.20.2 REVENUE ANALYSIS
18.20.3 PRODUCT PORTFOLIO
18.20.4 RECENT DEVELOPMENT
18.21 TEVA PHARMACEUTICAL INDUSTRIES LTD. (2022)
18.21.1 COMPANY SNAPSHOT
18.21.2 REVENUE ANALYSIS
18.21.3 PRODUCT PORTFOLIO
18.21.4 RECENT DEVELOPMENT
18.22 ZYDUS PHARMACEUTICALS, INC. (2022)
18.22.1 COMPANY SNAPSHOT
18.22.2 REVENUE ANALYSIS
18.22.3 PRODUCT PORTFOLIO
18.22.4 RECENT DEVELOPMENTS
19 QUESTIONNAIRE
20 RELATED REPORTS
Lista de Tablas
TABLE 1 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY INDICATION, 2021- 2030 (USD MILLION)
TABLE 2 NORTH AMERICA INFLUENZA IN ANTIVIRAL DRUGS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 3 NORTH AMERICA INFLUENZA IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 4 NORTH AMERICA NEURAMINIDASE INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 5 NORTH AMERICA M2 INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 6 NORTH AMERICA M2 INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 7 NORTH AMERICA HUMAN IMMUNODEFICIENCY VIRUS (HIV) IN ANTIVIRAL DRUGS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 8 NORTH AMERICA HUMAN IMMUNODEFICIENCY VIRUS (HIV) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 9 NORTH AMERICA REVERSE TRANSCRIPTASE INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 10 NORTH AMERICA NUCLEOSIDE (NRTIS) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 11 NORTH AMERICA NONNUCLEOSIDE (NNRTIS) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 12 NORTH AMERICA INTEGRASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 13 NORTH AMERICA NUCLEOTIDE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 14 NORTH AMERICA INTERFERONS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 15 NORTH AMERICA GP41 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 16 NORTH AMERICA PROTEASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 17 NORTH AMERICA HEPATITIS C VIRUS IN ANTIVIRAL DRUGS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 18 NORTH AMERICA HEPATITIS C VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 19 NORTH AMERICA NS5B POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 20 NORTH AMERICA NS3/4A PROTEASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 21 NORTH AMERICA NS5A PHOSPHOPROTEIN IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 22 NORTH AMERICA NEURAMINIDASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 23 NORTH AMERICA RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 24 NORTH AMERICA MATRIX PROTEIN 2 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 25 NORTH AMERICA HERPES SIMPLEX VIRUS IN ANTIVIRAL DRUGS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 26 NORTH AMERICA HERPES SIMPLEX VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 27 NORTH AMERICA DNA POLYMERASE UL30 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 28 NORTH AMERICA ENVELOPE PROTEINS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 29 NORTH AMERICA HUMAN CYTOMEGALOVIRUS (HCMV) IN ANTIVIRAL DRUGS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 30 NORTH AMERICA HUMAN CYTOMEGALOVIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 31 NORTH AMERICA VARICELLA-ZOSTER VIRUS (VZV) IN ANTIVIRAL DRUGS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 32 NORTH AMERICA DNA POLYMERASE ADEFOVIR IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 33 NORTH AMERICA HEPATITIS B VIRUS IN ANTIVIRAL DRUGS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 34 NORTH AMERICA DNA POLYMERASE ADEFOVIR IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 35 NORTH AMERICA RESPIRATORY SYNCYTIAL VIRUS IN ANTIVIRAL DRUGS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 36 NORTH AMERICA RESPIRATORY SYNCYTICAL VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 37 NORTH AMERICA RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 38 NORTH AMERICA FUSION GLYCOPROTEIN IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 39 NORTH AMERICA CORONAVIRUS INFECTION IN ANTIVIRAL DRUGS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 40 NORTH AMERICA OTHERS IN ANTIVIRAL DRUGS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 41 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021- 2030 (USD MILLION)
TABLE 42 NORTH AMERICA GERIATRIC IN ANTIVIRAL DRUGS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 43 NORTH AMERICA GERIATRIC IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 44 NORTH AMERICA CHILD IN ANTIVIRAL DRUGS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 45 NORTH AMERICA CHILD IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 46 NORTH AMERICA ADULT IN ANTIVIRAL DRUGS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 47 NORTH AMERICA ADULT IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 48 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY PRODUCT, 2021- 2030 (USD MILLION)
TABLE 49 NORTH AMERICA ORAL IN ANTIVIRAL DRUGS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 50 NORTH AMERICA ORAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 51 NORTH AMERICA SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 52 NORTH AMERICA SEMI-SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 53 NORTH AMERICA LIQUID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 54 NORTH AMERICA TOPICAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 55 NORTH AMERICA TOPICAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 56 NORTH AMERICA SEMI-SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 57 NORTH AMERICA LIQUID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 58 NORTH AMERICA SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 59 NORTH AMERICA PARENTERAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 60 NORTH AMERICA PARENTERAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 61 NORTH AMERICA CONVENTIONAL DRUG DELIVERY FORMULATIONS IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 62 NORTH AMERICA NOVEL DRUG DELIVERY FORMULATIONS IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 63 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021- 2030 (USD MILLION)
TABLE 64 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY END USER, 2021- 2030 (USD MILLION)
TABLE 65 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2021- 2030 (USD MILLION)
TABLE 66 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY COUNTRY, 2021-2030 (USD MILLION)
TABLE 67 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 68 NORTH AMERICA INFLUENZA IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 69 NORTH AMERICA NEURAMINIDASE INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 70 NORTH AMERICA M2 INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 71 NORTH AMERICA RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 72 NORTH AMERICA HUMAN IMMUNODEFICIENCY VIRUS (HIV) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 73 NORTH AMERICA REVERSE TRANSCRIPTASE INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 74 NORTH AMERICA NUCLEOSIDE (NRTIS) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 75 NORTH AMERICA NONNUCLEOSIDE (NNRTIS) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 76 NORTH AMERICA INTEGRASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 77 NORTH AMERICA NUCLEOTIDE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 78 NORTH AMERICA INTERFERONS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 79 NORTH AMERICA GP41 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 80 NORTH AMERICA PROTEASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 81 NORTH AMERICA HEPATITIS C VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 82 NORTH AMERICA NS5B POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 83 NORTH AMERICA NS3/4A PROTEASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 84 NORTH AMERICA NS5A PHOSPHOPROTEIN IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 85 NORTH AMERICA NEURAMINIDASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 86 NORTH AMERICA RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 87 NORTH AMERICA MATRIX PROTEIN 2 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 88 NORTH AMERICA HERPES SIMPLEX VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 89 NORTH AMERICA DNA POLYMERASE UL30 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 90 NORTH AMERICA ENVELOPE PROTEINS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 91 NORTH AMERICA HUMAN CYTOMEGALOVIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 92 NORTH AMERICA VARICELLA-ZOSTER VIRUS (VZV) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 93 NORTH AMERICA DNA POLYMERASE ADEFOVIR IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 94 NORTH AMERICA RESPIRATORY SYNCYTICAL VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 95 NORTH AMERICA RESPIRATORY SYNCYTICAL VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 96 NORTH AMERICA RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 97 NORTH AMERICA FUSION GLYCOPROTEIN IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 98 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 99 NORTH AMERICA GERIATRIC IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 100 NORTH AMERICA CHILD IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 101 NORTH AMERICA ADULT IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 102 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 103 NORTH AMERICA ORAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 104 NORTH AMERICA SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 105 NORTH AMERICA SEMI-SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 106 NORTH AMERICA LIQUID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 107 NORTH AMERICA TOPICAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 108 NORTH AMERICA SEMI-SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 109 NORTH AMERICA LIQUID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 110 NORTH AMERICA SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 111 NORTH AMERICA PARENTERAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 112 NORTH AMERICA CONVENTIONAL DRUG DELIVERY FORMULATIONS IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 113 NORTH AMERICA NOVEL DRUG DELIVERY FORMULATIONS IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 114 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 115 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 116 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 117 U.S. ANTIVIRAL DRUGS MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 118 U.S. INFLUENZA IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 119 U.S. NEURAMINIDASE INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 120 U.S. M2 INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 121 U.S. RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 122 U.S. HUMAN IMMUNODEFICIENCY VIRUS (HIV) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 123 U.S. REVERSE TRANSCRIPTASE INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 124 U.S. NUCLEOSIDE (NRTIS) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 125 U.S. NONNUCLEOSIDE (NNRTIS) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 126 U.S. INTEGRASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 127 U.S. NUCLEOTIDE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 128 U.S. INTERFERONS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 129 U.S. GP41 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 130 U.S. PROTEASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 131 U.S. HEPATITIS C VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 132 U.S. NS5B POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 133 U.S. NS3/4A PROTEASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 134 U.S. NS5A PHOSPHOPROTEIN IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 135 U.S. NEURAMINIDASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 136 U.S. RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 137 U.S. MATRIX PROTEIN 2 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 138 U.S. HERPES SIMPLEX VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 139 U.S. DNA POLYMERASE UL30 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 140 U.S. ENVELOPE PROTEINS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 141 U.S. HUMAN CYTOMEGALOVIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 142 U.S. VARICELLA-ZOSTER VIRUS (VZV) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 143 U.S. DNA POLYMERASE ADEFOVIR IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 144 U.S. RESPIRATORY SYNCYTICAL VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 145 U.S. RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 146 U.S. FUSION GLYCOPROTEIN IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 147 U.S. ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 148 U.S. GERIATRIC IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 149 U.S. CHILD IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 150 U.S. ADULT IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 151 U.S. ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 152 U.S. ORAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 153 U.S. SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 154 U.S. SEMI-SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 155 U.S. LIQUID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 156 U.S. TOPICAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 157 U.S. SEMI-SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 158 U.S. LIQUID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 159 U.S. SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 160 U.S. PARENTERAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 161 U.S.CONVENTIONAL DRUG DELIVERY FORMULATIONS IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 162 U.S. NOVEL DRUG DELIVERY FORMULATIONS IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 163 U.S. ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 164 U.S. ANTIVIRAL DRUGS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 165 U.S. ANTIVIRAL DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 166 CANADA ANTIVIRAL DRUGS MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 167 CANADA INFLUENZA IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 168 CANADA NEURAMINIDASE INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 169 CANADA M2 INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 170 CANADA RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 171 CANADA HUMAN IMMUNODEFICIENCY VIRUS (HIV) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 172 CANADA REVERSE TRANSCRIPTASE INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 173 CANADA NUCLEOSIDE (NRTIS) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 174 CANADA NONNUCLEOSIDE (NNRTIS) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 175 CANADA INTEGRASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 176 CANADA NUCLEOTIDE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 177 CANADA INTERFERONS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 178 CANADA GP41 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 179 CANADA PROTEASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 180 CANADA HEPATITIS C VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 181 CANADA NS5B POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 182 CANADA NS3/4A PROTEASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 183 CANADA NS5A PHOSPHOPROTEIN IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 184 CANADA NEURAMINIDASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 185 CANADA RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 186 CANADA MATRIX PROTEIN 2 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 187 CANADA HERPES SIMPLEX VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 188 CANADA DNA POLYMERASE UL30 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 189 CANADA ENVELOPE PROTEINS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 190 CANADA HUMAN CYTOMEGALOVIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 191 CANADA VARICELLA-ZOSTER VIRUS (VZV) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 192 CANADA RESPIRATORY SYNCYTICAL VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 193 CANADA RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 194 CANADA FUSION GLYCOPROTEIN IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 195 CANADA ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 196 CANADA GERIATRIC IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 197 CANADA CHILD IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 198 CANADA ADULT IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 199 CANADA ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 200 CANADA ORAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 201 CANADA SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 202 CANADA SEMI-SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 203 CANADA LIQUID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 204 CANADA TOPICAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 205 CANADA SEMI-SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 206 CANADA LIQUID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 207 CANADA SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 208 CANADA PARENTERAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 209 CANADA CONVENTIONAL DRUG DELIVERY FORMULATIONS IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 210 CANADA NOVEL DRUG DELIVERY FORMULATIONS IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 211 CANADA ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 212 CANADA ANTIVIRAL DRUGS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 213 CANADA ANTIVIRAL DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 214 MEXICO ANTIVIRAL DRUGS MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 215 MEXICO INFLUENZA IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 216 MEXICO NEURAMINIDASE INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 217 MEXICO M2 INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 218 MEXICO RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 219 MEXICO HUMAN IMMUNODEFICIENCY VIRUS (HIV) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 220 MEXICO REVERSE TRANSCRIPTASE INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 221 MEXICO NUCLEOSIDE (NRTIS) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 222 MEXICO NONNUCLEOSIDE (NNRTIS) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 223 MEXICO INTEGRASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 224 MEXICO NUCLEOTIDE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 225 MEXICO INTERFERONS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 226 MEXICO GP41 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 227 MEXICO PROTEASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 228 MEXICO HEPATITIS C VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 229 MEXICO NS5B POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 230 MEXICO NS3/4A PROTEASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 231 MEXICO NS5A PHOSPHOPROTEIN IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 232 MEXICO NEURAMINIDASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 233 MEXICO RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 234 MEXICO MATRIX PROTEIN 2 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 235 MEXICO HERPES SIMPLEX VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 236 MEXICO DNA POLYMERASE UL30 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 237 MEXICO ENVELOPE PROTEINS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 238 MEXICO HUMAN CYTOMEGALOVIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 239 MEXICO VARICELLA-ZOSTER VIRUS (VZV) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 240 MEXICO DNA POLYMERASE ADEFOVIR IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 241 MEXICO RESPIRATORY SYNCYTICAL VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 242 MEXICO RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 243 MEXICO FUSION GLYCOPROTEIN IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 244 MEXICO ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 245 MEXICO GERIATRIC IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 246 MEXICO CHILD IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 247 MEXICO ADULT IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 248 MEXICO ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 249 MEXICO ORAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 250 MEXICO SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 251 MEXICO SEMI-SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 252 MEXICO LIQUID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 253 MEXICO TOPICAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 254 MEXICO SEMI-SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 255 MEXICO LIQUID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 256 MEXICO SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 257 MEXICO PARENTERAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 258 MEXICO CONVENTIONAL DRUG DELIVERY FORMULATIONS IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 259 MEXICO NOVEL DRUG DELIVERY FORMULATIONS IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 260 MEXICO ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 261 MEXICO ANTIVIRAL DRUGS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 262 MEXICO ANTIVIRAL DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
Lista de figuras
FIGURE 1 NORTH AMERICA ANTIVIRAL DRUGS: SEGMENTATION
FIGURE 2 NORTH AMERICA ANTIVIRAL DRUGS MARKET: DATA TRIANGULATION
FIGURE 3 NORTH AMERICA ANTIVIRAL DRUGS MARKET: DROC ANALYSIS
FIGURE 4 NORTH AMERICA ANTIVIRAL DRUGS MARKET: NORTH AMERICA VS REGIONAL MARKET ANALYSIS
FIGURE 5 NORTH AMERICA ANTIVIRAL DRUGS MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 NORTH AMERICA ANTIVIRAL DRUGS MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 NORTH AMERICA ANTIVIRAL DRUGS MARKET: DBMR MARKET POSITION GRID
FIGURE 8 NORTH AMERICA ANTIVIRAL DRUGS MARKET: MARKET TESTING TYPE COVERAGE GRID
FIGURE 9 NORTH AMERICA ANTIVIRAL DRUGS MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 NORTH AMERICA ANTIVIRAL DRUGS MARKET: SEGMENTATION
FIGURE 11 THE RISING PREVALENCE OF VIRAL INFECTIONS IS EXPECTED TO DRIVE THE NORTH AMERICA ANTIVIRAL DRUGS MARKET IN THE FORECAST PERIOD
FIGURE 12 THE INFLUENZA SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE NORTH AMERICA ANTIVIRAL DRUGS MARKET IN 2023 & 2030
FIGURE 13 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE NORTH AMERICA ANTIVIRAL DRUGS MARKET
FIGURE 14 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY INDICATION , 2022
FIGURE 15 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY INDICATION , 2023-2030 (USD MILLION)
FIGURE 16 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY INDICATION , CAGR (2023-2030)
FIGURE 17 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY INDICATION , LIFELINE CURVE
FIGURE 18 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY PATIENT TYPE, 2022
FIGURE 19 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY PATIENT TYPE, 2023-2030 (USD MILLION)
FIGURE 20 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY PATIENT TYPE, CAGR (2023-2030)
FIGURE 21 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY PATIENT TYPE, LIFELINE CURVE
FIGURE 22 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY PRODUCT, 2022
FIGURE 23 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY PRODUCT, 2023-2030 (USD MILLION)
FIGURE 24 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY PRODUCT, CAGR (2023-2030)
FIGURE 25 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY PRODUCT, LIFELINE CURVE
FIGURE 26 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY DRUG TYPE, 2022
FIGURE 27 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY DRUG TYPE, 2023-2030 (USD MILLION)
FIGURE 28 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY DRUG TYPE, CAGR (2023-2030)
FIGURE 29 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY DRUG TYPE, LIFELINE CURVE
FIGURE 30 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY END USER, 2022
FIGURE 31 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY END USER, 2023-2030 (USD MILLION)
FIGURE 32 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY END USER, CAGR (2023-2030)
FIGURE 33 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY END USER, LIFELINE CURVE
FIGURE 34 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY DISTRIBUTION CHANNEL, 2022
FIGURE 35 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY DISTRIBUTION CHANNEL, 2023-2030 (USD MILLION)
FIGURE 36 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY DISTRIBUTION CHANNEL, CAGR (2023-2030)
FIGURE 37 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 38 NORTH AMERICA ANTIVIRAL DRUGS MARKET: SNAPSHOT (2022)
FIGURE 39 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY COUNTRY (2022)
FIGURE 40 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY COUNTRY (2023 & 2030)
FIGURE 41 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY COUNTRY (2022 & 2030)
FIGURE 42 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY INDICATION (2023-2030)
FIGURE 43 NORTH AMERICA ANTIVIRAL DRUGS MARKET: COMPANY SHARE 2022 (%)
Metodología de investigación
La recopilación de datos y el análisis del año base se realizan utilizando módulos de recopilación de datos con muestras de gran tamaño. La etapa incluye la obtención de información de mercado o datos relacionados a través de varias fuentes y estrategias. Incluye el examen y la planificación de todos los datos adquiridos del pasado con antelación. Asimismo, abarca el examen de las inconsistencias de información observadas en diferentes fuentes de información. Los datos de mercado se analizan y estiman utilizando modelos estadísticos y coherentes de mercado. Además, el análisis de la participación de mercado y el análisis de tendencias clave son los principales factores de éxito en el informe de mercado. Para obtener más información, solicite una llamada de un analista o envíe su consulta.
La metodología de investigación clave utilizada por el equipo de investigación de DBMR es la triangulación de datos, que implica la extracción de datos, el análisis del impacto de las variables de datos en el mercado y la validación primaria (experto en la industria). Los modelos de datos incluyen cuadrícula de posicionamiento de proveedores, análisis de línea de tiempo de mercado, descripción general y guía del mercado, cuadrícula de posicionamiento de la empresa, análisis de patentes, análisis de precios, análisis de participación de mercado de la empresa, estándares de medición, análisis global versus regional y de participación de proveedores. Para obtener más información sobre la metodología de investigación, envíe una consulta para hablar con nuestros expertos de la industria.
Personalización disponible
Data Bridge Market Research es líder en investigación formativa avanzada. Nos enorgullecemos de brindar servicios a nuestros clientes existentes y nuevos con datos y análisis que coinciden y se adaptan a sus objetivos. El informe se puede personalizar para incluir análisis de tendencias de precios de marcas objetivo, comprensión del mercado de países adicionales (solicite la lista de países), datos de resultados de ensayos clínicos, revisión de literatura, análisis de mercado renovado y base de productos. El análisis de mercado de competidores objetivo se puede analizar desde análisis basados en tecnología hasta estrategias de cartera de mercado. Podemos agregar tantos competidores sobre los que necesite datos en el formato y estilo de datos que esté buscando. Nuestro equipo de analistas también puede proporcionarle datos en archivos de Excel sin procesar, tablas dinámicas (libro de datos) o puede ayudarlo a crear presentaciones a partir de los conjuntos de datos disponibles en el informe.