North America Point Care Testing Poct Market
Tamaño del mercado en miles de millones de dólares
Tasa de crecimiento anual compuesta (CAGR) :
% 
 USD
25.20 Billion
USD
52.47 Billion
2025
2033
USD
25.20 Billion
USD
52.47 Billion
2025
2033
| 2026 –2033 | |
| USD 25.20 Billion | |
| USD 52.47 Billion | |
|
|
|
|
Mercado norteamericano de pruebas en el punto de atención (POCT), por tipo de producto (productos para el control de glucosa, productos para enfermedades infecciosas, productos para el control cardiometabólico, productos para pruebas de embarazo y fertilidad, productos para pruebas hematológicas, productos para el control de la coagulación, productos para pruebas de drogas de abuso (DOA), productos para análisis de orina, productos para pruebas de colesterol, productos para pruebas de marcadores tumorales/cáncer, productos para pruebas de heces ocultas y otros), por plataforma (ensayos de flujo lateral/pruebas de inmunocromatografía, inmunoensayos, tiras reactivas, diagnóstico molecular, ensayos de química clínica, microfluídica, hematología, otros), por aplicación (glucosa en sangre, enfermedades infecciosas, control de signos vitales, control cardíaco, coagulación, hematología, control no invasivo de SpO2, transfusión sanguínea, control no invasivo de PCO2, análisis de sangre completa, otros), por Modo de prescripción (Pruebas con receta y pruebas de venta libre, por usuario final (hospitales, atención domiciliaria, clínicas, laboratorios, centros de diagnóstico, laboratorios de patología, centros de cirugía ambulatoria, centros de atención para personas mayores, otros), por canal de distribución (licitación directa, ventas minoristas, ventas en línea y otros), país (EE. UU., Canadá, México): tendencias de la industria y pronóstico hasta 2033
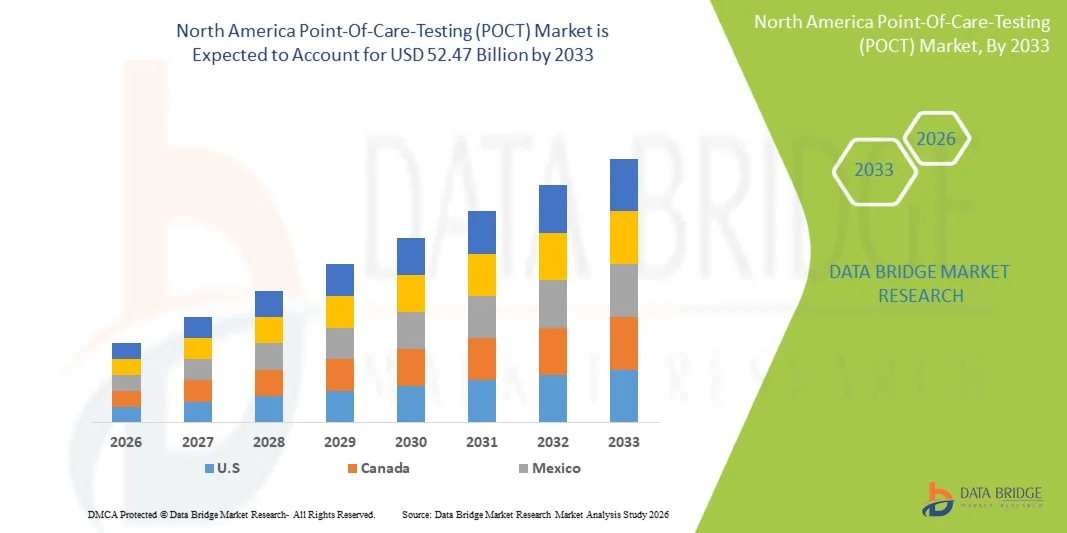
Tamaño del mercado de pruebas en el punto de atención (POCT) en América del Norte
- El tamaño del mercado de pruebas en el punto de atención (POCT) de América del Norte se valoró en USD 25,20 mil millones en 2025 y se espera que alcance los USD 52,47 mil millones para 2033 , con una CAGR del 9,6% durante el período de pronóstico.
- El crecimiento del mercado norteamericano de pruebas en el punto de atención (POCT) se debe principalmente a la creciente demanda de soluciones de diagnóstico rápido que permitan la toma de decisiones clínicas inmediatas y reduzcan la dependencia de las pruebas de laboratorio centralizadas. Los dispositivos POCT permiten tiempos de respuesta más rápidos, la detección temprana de enfermedades y mejores resultados para los pacientes, aspectos fundamentales en la atención de urgencias, la atención médica remota y la monitorización domiciliaria.
- La creciente prevalencia de enfermedades crónicas como la diabetes, los trastornos cardiovasculares, las enfermedades infecciosas y las enfermedades respiratorias está incrementando significativamente la adopción de dispositivos POCT para la monitorización y el tratamiento en tiempo real. Además, los avances en tecnologías de diagnóstico, microfluídica, biosensores y plataformas de atención médica conectadas están mejorando la precisión, la portabilidad y la capacidad de integración de datos, impulsando el crecimiento del mercado.
Análisis del mercado de pruebas en el punto de atención (POCT) en América del Norte
- El mercado norteamericano de pruebas en el punto de atención (POCT) es un segmento en rápida expansión de la industria del diagnóstico, que ofrece resultados inmediatos directamente en las instalaciones del paciente, como clínicas, hospitales, ambulancias y domicilios. Los dispositivos POCT permiten la detección y el monitoreo rápidos de afecciones como diabetes, enfermedades infecciosas, embarazo, marcadores cardíacos, desequilibrio electrolítico y parámetros de coagulación, lo que permite una mayor eficiencia clínica y una menor carga para el sistema sanitario.
- La creciente demanda de tecnologías de diagnóstico portátiles y fáciles de usar se debe principalmente a la creciente prevalencia de enfermedades crónicas, las necesidades de atención de emergencia y la expansión de la monitorización domiciliaria de pacientes. Además, la integración de plataformas de salud digital, diagnósticos basados en teléfonos inteligentes, detección con IA y conectividad inalámbrica facilita una mejor trazabilidad de los datos y la gestión remota de pacientes, impulsando aún más el crecimiento del mercado.
- Se espera que EE. UU. domine el mercado de pruebas en el punto de atención (POCT) de América del Norte con la mayor participación en los ingresos de alrededor del 83,12 % en 2026, impulsado por la fuerte presencia de actores clave del mercado, el alto gasto en atención médica, la infraestructura médica avanzada y la creciente adopción de tecnologías de diagnóstico innovadoras en hospitales y entornos de atención domiciliaria.
- Se espera que el segmento de productos de monitoreo de glucosa domine el mercado de POCT de América del Norte con una participación importante de más del 38,86 % en 2026, impulsado por la creciente población diabética, la preferencia por dispositivos de monitoreo en tiempo real y el uso creciente de medidores portátiles de glucosa en sangre y herramientas de monitoreo continuo de glucosa entre pacientes de atención domiciliaria.
Alcance del informe y segmentación del mercado de pruebas en el punto de atención (POCT) en América del Norte
|
Atributos |
Análisis clave del mercado de pruebas en el punto de atención (POCT) en América del Norte |
|
Segmentos cubiertos |
|
|
Países cubiertos |
América del norte
|
|
Actores clave del mercado |
|
|
Oportunidades de mercado |
|
|
Conjuntos de información de datos de valor añadido |
Además de los conocimientos sobre escenarios de mercado como valor de mercado, tasa de crecimiento, segmentación, cobertura geográfica y actores principales, los informes de mercado seleccionados por Data Bridge Market Research también incluyen seguimiento de innovación y análisis estratégico, avances tecnológicos, escenario de cambio climático, análisis de la cadena de suministro, análisis de la cadena de valor, criterios de selección de proveedores, análisis PESTLE, análisis de Porter, análisis de patentes, análisis del ecosistema de la industria, cobertura de materia prima, aranceles y su impacto en el mercado, cobertura regulatoria, comportamiento de compra del consumidor, perspectiva de marca, desglose del análisis de costos y marco regulatorio. |
Tendencias del mercado de pruebas en el punto de atención (POCT) en América del Norte
“Avances tecnológicos y expansión funcional mediante I+D e integración digital ”
- Una tendencia importante y de rápido crecimiento en el mercado norteamericano de pruebas en el punto de atención (POCT) es el creciente enfoque en la innovación, la I+D y las tecnologías de diagnóstico avanzadas, destinadas a mejorar la precisión, la velocidad, la portabilidad y la toma de decisiones en tiempo real. Ante la creciente demanda de pruebas descentralizadas y centradas en el paciente, los profesionales sanitarios, las empresas de diagnóstico y los fabricantes de dispositivos médicos están invirtiendo fuertemente en plataformas POCT de última generación que facilitan diagnósticos de precisión y una mejor conectividad en todos los centros de atención.
- Empresas líderes como Abbott, Roche, Siemens Healthineers, Danaher y Thermo Fisher Scientific están acelerando sus esfuerzos de investigación para desarrollar dispositivos miniaturizados, tecnologías de laboratorio en chip y sistemas de análisis basados en IA capaces de ofrecer resultados con calidad de laboratorio en el momento preciso. Estos avances tecnológicos también incluyen la manipulación automatizada de muestras, una mayor sensibilidad de los biosensores y la capacidad de realizar pruebas multiplex, lo que permite la detección simultánea de múltiples biomarcadores.
- En el diagnóstico de enfermedades infecciosas, se han desarrollado extensas iniciativas de I+D para producir soluciones rápidas de pruebas moleculares con alta precisión, tiempos de respuesta más cortos y adaptadas a situaciones de emergencia y brotes. Los dispositivos POCT diseñados para la gripe, la COVID-19, las infecciones respiratorias, el VIH y la sepsis se están optimizando con tecnologías de microfluídica y amplificación isotérmica, lo que permite realizar pruebas fiables fuera de laboratorios centralizados.
- En el manejo de enfermedades crónicas, la innovación en la monitorización de glucosa, las pruebas de biomarcadores cardíacos, las pruebas de coagulación y el análisis de la función renal facilita la monitorización continua y proporciona datos en tiempo real para la intervención clínica. Las empresas están integrando aplicaciones móviles, informes en la nube y dispositivos portátiles de diagnóstico para facilitar la monitorización domiciliaria fluida y la conectividad remota de telesalud.
- El mercado también está experimentando una expansión en el diagnóstico personalizado, con el desarrollo de soluciones POCT para apoyar la detección de oncología, fertilidad, trastornos gastrointestinales y enfermedades metabólicas. Estas innovaciones buscan permitir una detección más temprana, decisiones terapéuticas más rápidas y mejores resultados clínicos, especialmente en entornos ambulatorios y de atención domiciliaria.
- Además, la integración de dispositivos conectados, el análisis de datos digitales y la interpretación basada en IA está permitiendo ecosistemas POCT más inteligentes que optimizan la automatización del flujo de trabajo y reducen el error humano. Estos avances están transformando el POCT en una herramienta de diagnóstico multifuncional e inteligente capaz de respaldar la atención preventiva, la medicina de precisión y la gestión de la salud poblacional.
- Este entorno en rápida evolución, impulsado por la innovación, está transformando el mercado de POCT, orientando la industria hacia sistemas de diagnóstico portátiles, integrados y centrados en el paciente. A medida que los sistemas de salud de Norteamérica priorizan la eficiencia, la accesibilidad y la sostenibilidad, se espera que la transformación digital impulsada por la I+D abra nuevas aplicaciones y amplíe la penetración en el mercado, tanto en regiones desarrolladas como emergentes.
Dinámica del mercado de pruebas en el punto de atención (POCT) en América del Norte
Conductor
Creciente demanda de soluciones de diagnóstico rápidas, descentralizadas y centradas en el paciente
- Un factor clave que acelera el crecimiento del mercado norteamericano de pruebas en el punto de atención (POCT) es la creciente necesidad de herramientas de diagnóstico rápidas, accesibles y descentralizadas que mejoren la toma de decisiones clínicas y los resultados de los pacientes. A medida que los sistemas de salud evolucionan hacia modelos basados en el valor y centrados en el paciente, las pruebas POCT ofrecen resultados inmediatos en el centro de atención o cerca de él, lo que reduce la dependencia de laboratorios centralizados y permite un diagnóstico temprano y tratamientos oportunos.
- Empresas líderes del sector, como Abbott, Roche, Siemens Healthineers y Danaher, están expandiendo activamente sus inversiones en I+D para desarrollar dispositivos POCT portátiles, de alta precisión y con conexión digital, capaces de facilitar el diagnóstico en tiempo real en hospitales, clínicas, urgencias y entornos de atención domiciliaria. Estas innovaciones se alinean con la creciente demanda de soluciones integradas de salud digital, la expansión de la telemedicina y la interpretación de resultados asistida por IA.
- En el manejo de enfermedades crónicas, los dispositivos POCT para la monitorización de glucosa, biomarcadores cardíacos, pruebas de coagulación y evaluación renal están experimentando un rápido crecimiento debido a la creciente prevalencia de diabetes, enfermedades cardiovasculares y trastornos del estilo de vida en Norteamérica. De igual manera, los sistemas POCT para enfermedades infecciosas como la COVID-19, la influenza, la sepsis, la malaria y el VIH se están convirtiendo en herramientas cruciales para el control de brotes, especialmente en regiones con recursos limitados.
- El enfoque creciente en el monitoreo remoto y domiciliario de pacientes también está impulsando su adopción, respaldado por el desarrollo de diagnósticos habilitados para teléfonos inteligentes, soluciones de pruebas portátiles, plataformas basadas en la nube y capacidades de informes remotos que mejoran la conectividad clínica y la continuidad de la atención.
- Con la modernización de la infraestructura sanitaria, el creciente énfasis en la rapidez de diagnóstico y las fuertes inversiones públicas y privadas, la POCT se perfila como un componente esencial para la prestación de servicios de salud en el futuro. A medida que la eficiencia y la accesibilidad se convierten en elementos centrales de la reforma sanitaria en Norteamérica, se prevé un aumento significativo de la demanda de sistemas POCT innovadores, precisos y orientados al paciente.
Restricción/Desafío
“ Altos costos, complejidades regulatorias y preocupaciones sobre precisión y estandarización ”
- A pesar del sólido crecimiento del mercado, el mercado norteamericano de pruebas en el punto de atención (POCT) se enfrenta a importantes desafíos relacionados con los altos costos de equipos y pruebas, los rigurosos procesos de aprobación regulatoria y la variabilidad en la precisión diagnóstica entre las diferentes plataformas de dispositivos. Garantizar un rendimiento analítico consistente, comparable a los estándares de laboratorio centralizados, sigue siendo un obstáculo clave, especialmente a medida que las pruebas en el punto de atención se expanden a los segmentos de cuidados intensivos y pruebas de alta complejidad.
- Obtener las aprobaciones de organismos reguladores como la FDA, la EMA y otras autoridades nacionales requiere una exhaustiva validación clínica, cumplimiento de la calidad y vigilancia poscomercialización. Estos procesos pueden retrasar significativamente los plazos de lanzamiento de productos y aumentar los gastos de desarrollo para los fabricantes, especialmente en tecnologías de diagnóstico emergentes como la POCT molecular y las pruebas multiplex.
- Las limitaciones de costos también limitan la adopción en regiones en desarrollo, donde los presupuestos sanitarios limitados y los sistemas de reembolso inadecuados restringen su implementación generalizada. Además, los desafíos operativos, como los requisitos de mantenimiento, la capacitación de los usuarios y la integración del flujo de trabajo, pueden dificultar la implementación a gran escala en hospitales y centros de diagnóstico.
- Las preocupaciones sobre la precisión, la fiabilidad y la estandarización de los resultados, en particular en pruebas de alta sensibilidad para enfermedades infecciosas y marcadores cardíacos, pueden generar dudas entre los profesionales clínicos y aumentar la dependencia de las pruebas confirmatorias centralizadas. Los problemas relacionados con la integración de datos y la ciberseguridad en las plataformas digitales de POCT también exigen mejores marcos tecnológicos e inversión.
- A medida que la tecnología POCT evoluciona, superar estos desafíos financieros, regulatorios y técnicos requerirá una amplia financiación para I+D, una mejor armonización de los estándares de diagnóstico en Norteamérica y una colaboración estratégica entre fabricantes de dispositivos, profesionales sanitarios y agencias gubernamentales de salud. Hasta entonces, estas barreras podrían seguir limitando el ritmo de adopción en ciertos segmentos del mercado y regiones.
Alcance del mercado de pruebas en el punto de atención (POCT) en América del Norte
El mercado está segmentado según el tipo de producto, la plataforma, la aplicación, el modo de prescripción, el usuario final y el canal de distribución.
- Por tipo de producto
Según el tipo de producto, el mercado norteamericano de pruebas en el punto de atención (POCT) se segmenta en productos para el control de glucosa, productos para el análisis de enfermedades infecciosas, productos para el control cardiometabólico, productos para el control de embarazo y fertilidad, productos para el análisis hematológico, productos para el control de la coagulación, productos para el análisis de drogas de abuso (DOA), productos para el análisis de orina, productos para el análisis de colesterol, productos para el análisis de marcadores tumorales/cancerosos, productos para el análisis de heces ocultas y otros. Se prevé que el segmento de productos para el control de la glucosa domine la mayor cuota de mercado, con un 38,86 %, en 2026, impulsado por la creciente incidencia de diabetes en Norteamérica, la creciente demanda de autocontrol domiciliario y los continuos avances tecnológicos en glucómetros y plataformas de salud digital conectadas.
- Por plataforma
Según la Plataforma, el mercado norteamericano de pruebas en el punto de atención (POCT) se segmenta en ensayos de flujo lateral/pruebas de inmunocromatografía, inmunoensayos, tiras reactivas, diagnóstico molecular, ensayos de química clínica, microfluídica, hematología y otros. Se prevé que los ensayos de flujo lateral/pruebas de inmunocromatografía dominen el mercado en 2026, gracias a su amplia adopción en el cribado de enfermedades infecciosas, su facilidad de uso, la rápida entrega de resultados, su bajo coste y su amplio uso durante brotes como la COVID-19, la gripe, la malaria y el dengue. Su idoneidad para entornos sanitarios descentralizados y remotos impulsa aún más el crecimiento del segmento.
- Por aplicación
Según la aplicación, el mercado norteamericano de pruebas en el punto de atención (POCT) se segmenta en glucemia, enfermedades infecciosas, monitorización de constantes vitales, monitorización cardíaca, coagulación, hematología, monitorización no invasiva de SpO2, transfusión sanguínea, monitorización no invasiva de PCO2, análisis de sangre completa y otros. Se prevé que la glucemia domine con la mayor cuota de mercado en 2026, impulsada por el aumento de la prevalencia de la diabetes, la creciente preferencia de los pacientes por los dispositivos de monitorización portátiles y la disponibilidad de glucómetros de alta precisión y fácil manejo, así como de tecnologías de monitorización continua de glucosa (MCG) que facilitan el control de la diabetes en tiempo real.
- Por modo de prescripción
Según el Modo de Prescripción, el mercado norteamericano de pruebas en el punto de atención (POCT) se segmenta en pruebas de venta libre y pruebas con receta. El segmento de pruebas de venta libre representó la mayor cuota de mercado en 2026, gracias a la creciente preferencia de los consumidores por las soluciones de autodiagnóstico, la creciente disponibilidad de kits de diagnóstico rápido y el creciente enfoque en la atención médica preventiva. Los dispositivos POCT de venta libre permiten a las personas controlar parámetros de salud como los niveles de glucosa, el embarazo, la fertilidad, las enfermedades infecciosas y los indicadores cardiovasculares sin supervisión clínica.
- Por el usuario final
En función del usuario final, el mercado norteamericano de pruebas en el punto de atención (POCT) se segmenta en hospitales, atención domiciliaria, clínicas, laboratorios, centros de diagnóstico, laboratorios de patología, centros de cirugía ambulatoria, centros de atención a personas mayores y otros. El segmento de hospitales representó la mayor cuota de mercado en 2026, impulsado por la alta demanda de pruebas diagnósticas rápidas para respaldar la atención de emergencias, cuidados intensivos y evaluaciones clínicas de rutina. Los dispositivos POCT, como glucómetros, analizadores de marcadores cardíacos, pruebas rápidas para enfermedades infecciosas, monitores de coagulación y analizadores de gases en sangre, se utilizan cada vez más en entornos hospitalarios para agilizar la toma de decisiones clínicas, reducir el tiempo de respuesta a los pacientes y mejorar la eficiencia del flujo de trabajo.
- Por canal de distribución
Según el canal de distribución, el mercado norteamericano de pruebas en el punto de atención (POCT) se segmenta en licitación directa, ventas minoristas, ventas en línea y otros. El segmento de licitación directa representó la mayor cuota de mercado en 2026, impulsado por la adquisición masiva de dispositivos POCT por parte de hospitales, laboratorios de diagnóstico y organismos de salud pública. Las licitaciones a gran escala garantizan un suministro constante de herramientas de diagnóstico esenciales, como monitores de glucosa, kits de pruebas rápidas de enfermedades infecciosas, analizadores de biomarcadores cardíacos y dispositivos de coagulación, necesarios para las pruebas de rutina y la atención de emergencias.
Análisis regional del mercado de pruebas en el punto de atención (POCT) en América del Norte
- Norteamérica dominó el mercado de pruebas en el punto de atención (POCT) en Norteamérica, con la mayor participación en ingresos, superior al 39,83 % en 2025, impulsada por la rápida expansión de la infraestructura sanitaria de la región, la creciente carga de enfermedades crónicas y la creciente adopción de tecnologías de diagnóstico descentralizadas. Países como Estados Unidos, Canadá y México están experimentando una fuerte demanda de kits de diagnóstico rápido para diabetes, enfermedades infecciosas, afecciones cardiovasculares y monitoreo domiciliario.
- La creciente inversión en salud digital, la expansión de la telemedicina y las iniciativas gubernamentales que apoyan el diagnóstico temprano aceleran aún más la implementación de pruebas POCT. Además, la gran población geriátrica y la creciente preferencia de los consumidores por pruebas médicas asequibles y rápidas están impulsando el crecimiento del mercado en toda la región.
Análisis del mercado de pruebas en el punto de atención (POCT) en Norteamérica y EE. UU.
Estados Unidos dominó el mercado norteamericano de pruebas POCT en 2025, gracias a su avanzada infraestructura sanitaria, su alta concienciación sobre la detección temprana de enfermedades y la creciente preferencia por las soluciones de diagnóstico domiciliario. La creciente prevalencia de diabetes, trastornos cardíacos, enfermedades respiratorias y afecciones infecciosas sigue impulsando la demanda de kits de pruebas rápidas. La sólida capacidad de I+D, la alta adopción de dispositivos de prueba conectados e integrados en teléfonos inteligentes y un entorno regulatorio consolidado contribuyen aún más a la expansión del mercado. Además, la transición hacia diagnósticos personalizados y en el punto de necesidad, junto con el auge de las clínicas minoristas y las pruebas en farmacias, continúa fortaleciendo el panorama de las pruebas POCT en EE. UU.
Cuota de mercado de pruebas en el punto de atención (POCT) en América del Norte
La industria de pruebas en el punto de atención (POCT) está liderada principalmente por empresas bien establecidas, entre las que se incluyen:
- Abbott Point of Care Inc. (EE. UU.)
- Sinocare Inc. (China)
- F. Hoffmann-La Roche Ltd (Suiza)
- Corporación Danaher (EE. UU.)
- Hologic, Inc. (EE. UU.)
- bioMérieux SA (Francia)
- Siemens Healthineers AG (Alemania)
- Thermo Fisher Scientific Inc. (EE. UU.)
- BD Veritor (Becton, Dickinson and Company) (EE. UU.)
- QuidelOrtho Corporation (EE. UU.)
- Bio-Rad Laboratories, Inc. (EE. UU.)
- Werfen (España)
- Sekisui Diagnostics (Japón)
- Trividia Health, Inc. (EE. UU.)
- Nova Biomedical Corporation (EE. UU.)
- Meridian Bioscience, Inc. (EE. UU.)
- Pfizer Inc. (EE. UU.)
- Shenzhen New Industry Biomedical Engineering Co., Ltd. (China)
- Corporación Sysmex (Japón)
- Wondfo (Guangzhou Wondfo Biotech Co., Ltd.) (China)
- QIAGEN NV (Alemania)
- Abaxis, Inc. (EE. UU.)
- Autobio Diagnostics Co., Ltd. (China)
- Getein Biotech, Inc. (China)
- Chembio Diagnostics, Inc. (EE. UU.)
- EKF Diagnostics Holdings plc (Reino Unido)
- Trinity Biotech plc (Irlanda)
- Diagnóstico PTS (EE. UU.)
- QuantuMDx Group Ltd. (Reino Unido)
- Binx Health (EE. UU.)
- Xiamen Boson Biotech Co., Ltd. (China)
- Accubiotech Co., Ltd. (China)
- Sienco, Inc. (EE. UU.)
- LamdaGen Corporation (EE. UU.)
Últimos avances en el mercado de pruebas en el punto de atención (POCT) en América del Norte
- En mayo de 2020, la prueba ID NOW COVID-19 de Abbott proporcionó resultados rápidos y fiables en minutos, lo que facilita el diagnóstico oportuno y reduce el riesgo de infección. Los estudios demuestran un excelente rendimiento en centros de atención de urgencia, con una sensibilidad ≥94,7 % y una especificidad ≥98,6 %. A pesar de las dificultades de un estudio de la Universidad de Nueva York, los datos reales respaldan su eficacia. Autorizada por la FDA para uso de emergencia, la prueba desempeña un papel crucial en la detección de la COVID-19.
- En septiembre de 2025, Dongguan E-Test Technology, filial de Sinocare, recibió la autorización 510(k) de la FDA para sus monitores de presión arterial inteligentes Multi-Series, lo que destaca su precisión, seguridad y capacidad inalámbrica. Los dispositivos ofrecen monitorización de grado médico, conectividad Bluetooth y alertas inteligentes, lo que refuerza la estrategia de expansión de Sinocare en Norteamérica y mejora su ecosistema de gestión de enfermedades crónicas en mercados internacionales, como Estados Unidos y Europa.
- En enero de 2025, Danaher Corporation formó una alianza de inversión con Innovaccer Inc., una empresa de inteligencia artificial para el sector sanitario. Esta colaboración busca acelerar la adopción de diagnósticos de precisión y atención basada en el valor, proporcionando a los profesionales sanitarios datos unificados de los pacientes y análisis avanzados, mejorando así los resultados de los pacientes mediante intervenciones personalizadas y oportunas.
- En noviembre de 2023, Binx Health se asoció con Fisher Healthcare para ampliar la distribución de binx io, una plataforma molecular para el punto de atención que detecta clamidia y gonorrea, aprobada por la FDA. Este sistema proporciona resultados con calidad de laboratorio en aproximadamente 30 minutos, lo que mejora la rapidez del diagnóstico y permite a los profesionales clínicos realizar pruebas y tratar a los pacientes en una sola consulta, optimizando así el acceso a la atención médica.
SKU-
Obtenga acceso en línea al informe sobre la primera nube de inteligencia de mercado del mundo
- Panel de análisis de datos interactivo
- Panel de análisis de empresas para oportunidades con alto potencial de crecimiento
- Acceso de analista de investigación para personalización y consultas
- Análisis de la competencia con panel interactivo
- Últimas noticias, actualizaciones y análisis de tendencias
- Aproveche el poder del análisis de referencia para un seguimiento integral de la competencia
Tabla de contenido
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF THE NORTH AMERICA POINT-OF-CARE-TESTING (POCT) MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATIONS
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 DBMR TRIPOD DATA VALIDATION MODEL
2.5 MULTIVARIATE MODELLING
2.6 TYPE LIFELINE CURVE
2.7 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.8 DBMR MARKET POSITION GRID
2.9 MARKET END USER COVERAGE GRID
2.1 VENDOR SHARE ANALYSIS
2.11 SECONDARY SOURCES
2.12 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PORTER’S FIVE FORCES
4.2 PESTEL ANALYSIS
4.3 HEALTHCARE ECONOMY
4.3.1 HEALTHCARE EXPENDITURE
4.3.2 CAPITAL EXPENDITURE
4.3.3 CAPEX TRENDS
4.3.4 CAPEX ALLOCATION
4.3.5 FUNDING SOURCES
4.3.6 INDUSTRY BENCHMARKS
4.3.7 GDP RATIO IN OVERALL GDP
4.3.8 HEALTHCARE SYSTEM STRUCTURE
4.3.9 GOVERNMENT POLICIES
4.3.10 ECONOMIC DEVELOPMENT
4.4 REIMBURSEMENT FRAMEWORK
4.5 OPPORTUNITY MAP ANALYSIS
4.6 VALUE CHAIN ANALYSIS
4.7 MICRO AND MACRO ECONOMIC FACTORS
4.7.1 CURRENT MARKET PENETRATION
4.7.2 GROWTH PROSPECTS
4.7.3 KEY PRICING STRATEGIES
4.8 TECHNOLOGY ROADMAP: NORTH AMERICA POINT OF CARE TESTING
5 NORTH AMERICA POINT-OF-CARE TESTING (POCT) MARKET: REGULATIONS
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 RISING USE OF POC TESTING IN HEALTHCARE FACILITIES
6.1.2 RISING INCIDENCE OF SUBSTANCE ABUSE
6.1.3 INCREASED ADOPTION OF TELEMEDICINE
6.1.4 ADVANCEMENTS TECHNOLOGIES ENHANCING POC TESTING WITH BIOSENSORS AND MOBILE INTEGRATION
6.2 RESTRAINTS
6.2.1 DATA SECURITY AND PRIVACY CONCERNS
6.2.2 LACK OF ACCURACY AND TECHNICAL CHALLENGES
6.3 OPPORTUNITIES
6.3.1 RISING AWARENESS AND ADVOCACY FOR POINT-OF-CARE TESTING
6.3.2 STRATEGIC INITIATION AND DECISION TAKEN BY THE MARKET PLAYERS
6.3.3 EXPANDING PRODUCT RANGE FOR POINT-OF-CARE TESTING
6.4 CHALLENGES
6.4.1 LIMITED AWARENESS AND ACCEPTANCE
6.4.2 IMPACT OF HIGH MAINTENANCE COSTS THREATENING POINT-OF-CARE TESTING (POCT) SUSTAINABILITY IN LOW-RESOURCE SETTINGS
7 NORTH AMERICA POINT-OF-CARE TESTING (POCT) MARKET, BY PRODUCT TYPE
7.1 OVERVIEW
7.2 GLUCOSE MONITORING PRODUCTS
7.2.1 SELF-MONITORING OF BLOOD GLUCOSE (SMBG) DEVICES
7.2.1.1 Strips
7.2.1.2 Meters
7.2.1.3 Lancets and Lancing Devices
7.2.2 CONTINUOUS GLUCOSE MONITORING (CGM) SYSTEMS
7.3 INFECTIOUS DISEASE TESTING PRODUCTS
7.3.1 COVID-19
7.3.2 HIV TESTING PRODUCTS
7.3.2.1 Testing Reagents
7.3.2.2 Testing Equipment
7.3.3 RESPIRATORY INFECTION TESTING PRODUCTS
7.3.4 SEXUALLY TRANSMITTED DISEASES (STD) TESTING
7.3.4.1 NAAT-Based Systems
7.3.4.2 NON–NAAT-Based Systems
7.3.5 HEPATITIS C TESTING PRODUCTS
7.3.5.1 HCV Antibody Tests
7.3.5.2 HCV Viral Load Tests
7.3.6 INFLUENZA TESTING PRODUCTS
7.3.6.1 Traditional Diagnostic Test
7.3.6.2 Molecular Diagnostic Assay
7.3.6.2.1 Rapid Influenza Diagnostic Test (RIDT)
7.3.6.2.2 Direct Fluorescent Antibody Test (DFAT)
7.3.6.2.3 Viral Culture
7.3.6.2.4 Serological Assay
7.3.6.3 RT-PCR
7.3.6.4 Loop-Mediated Isothermal Amplification-Based Assay (LAMP)
7.3.6.5 Nucleic Acid Sequence-Based Amplification Test (NASBAT)
7.3.6.6 Simple Amplification-Based Assay (SAMBA)
7.3.6.7 Healthcare Associated Infection (HAI) Testing
7.3.6.8 Tropical Disease Testing Products
7.3.6.9 Other Infectious Disease Testing Products
7.4 CARDIOMETABOLIC MONITORING PRODUCTS
7.4.1 CARDIAC MARKER TESTING PRODUCTS
7.4.1.1 HSTNL
7.4.1.2 BNP
7.4.1.3 D-DIMER
7.4.1.4 CK-MB
7.4.1.5 Myoglobin
7.4.2 BLOOD GAS/ELECTROLYTE TESTING PRODUCTS
7.4.2.1 Blood Gas/Electrolyte Testing Consumables
7.4.2.2 Blood Gas/Electrolyte Testing Instruments
7.4.3 CARTRIDGES
7.4.4 REAGENTS
7.4.4.1 Portable
7.4.4.2 Benchtop
7.4.4.3 Combined Analyzers
7.4.4.4 Blood Gas Analyzers
7.4.4.5 Electrolyte Analyzers
7.4.4.6 Combined Analyzers
7.4.4.7 Blood Gas Analyzers
7.4.4.8 Electrolyte Analyzers
7.4.5 HBA1C TESTING PRODUCTS
7.4.5.1 HBA1C Testing Instruments
7.4.5.2 HBA1C Testing Consumables
7.4.5.3 POC Analyzer
7.4.5.4 ECG Device
7.4.5.5 Resting ECG Devices
7.4.5.6 Stress ECG Devices
7.4.5.7 Holter Monitors
7.5 PREGNANCY AND FERTILITY TESTING PRODUCTS
7.5.1 PREGNANCY TESTING PRODUCTS
7.5.1.1 Strips/ Dip Sticks and Cards
7.5.1.2 Mid Stream Devices
7.5.1.3 Cassettes
7.5.1.4 Digital Devices
7.5.1.5 Line-Indicator Devices
7.5.2 FERTILITY TESTING PRODUCTS
7.5.2.1 Luteinizing Hormone (LH) Urine Test
7.5.2.2 FSH Test
7.5.2.3 others
7.6 HAEMATOLOGY TESTING PRODUCTS
7.7 COAGULATION MONITORING PRODUCTS
7.7.1 ANTICOAGULATION MONITORING DEVICES
7.7.1.1 Prothrombin Time/International Normalized Ratio (PT-INR) Testing Devices
7.7.1.2 Activated Clotting Time (ACT)
7.7.1.3 Activated Partial Thromboplastin Time (APPT)
7.7.1.4 Platelet Function Monitoring Devices
7.7.1.5 Viscoelastic Coagulation Monitoring Devices
7.7.1.6 Rotational Thromboelastometry (ROTEM)
7.7.1.7 Thromboelastography (TEG)
7.7.1.8 Drug-Of-Abuse (DOA) Testing Products
7.7.2 DOA ANALYSERS
7.7.2.1 Immunoassays
7.7.2.2 Chromatographic Devices
7.7.2.3 Breath Analysers
7.7.3 RAPID TESTING DEVICES
7.7.3.1 Urine Testing Devices
7.7.3.2 Oral Fluid Testing Devices
7.7.3.4 Others
7.8 URINALYSIS TESTING PRODUCTS
7.8.1.1 POC Urine Strip Self-Testing
7.8.1.2 POC Urine Test Strip Professional Testing
7.9 CHOLESTEROL TESTING PRODUCTS
7.9.1.1 Testing Kits
7.9.1.2 Instruments
7.9.1.3 Table-Top Analyzers
7.9.1.4 Hand-Held Analyzers
7.1 TUMOR/CANCER MARKER TESTING PRODUCTS
7.11 FECAL OCCULT TESTING PRODUCTS
7.11.1.1 Guaiac FOB Stool Test
7.11.1.2 Lateral Flow Immuno-FOB Test
7.11.1.3 Immuno-FOB Agglutination Test
7.11.1.4 Immuno-FOB ELISA Test
7.12 OTHERS
8 NORTH AMERICA POINT-OF-CARE TESTING (POCT) MARKET, BY PLATFORM
8.1 OVERVIEW
8.2 LATERAL FLOW ASSAYS/IMMUNOCHROMATOGRAPHY TESTS
8.3 IMMUNOASSAYS
8.4 DIPSTICKS
8.5 MOLECULAR DIAGNOSTICS
8.6 CLINICAL CHEMISTRY ASSAYS
8.7 MICROFLUIDICS
8.8 HEMATOLOGY
8.9 OTHERS
9 NORTH AMERICA POINT-OF-CARE-TESTING (POCT) MARKET, BY APPLICATION
9.1 OVERVIEW
9.2 BLOOD GLUCOSE
9.3 INFECTIOUS DISEASES
9.3.1 COVID-19 TESTING
9.3.2 HIV TESTING
9.3.3 HEPATITIS C TESTING
9.3.4 INFLUENZA TESTING
9.3.5 TUBERCULOSIS TESTING
9.3.6 OTHERS
9.4 VITAL SIGN MONITORING
9.5 CARDIAC MONITORING
9.6 COAGULATION
9.7 HAEMATOLOGY
9.8 NON- INVASIVE SPO2 MONITORING
9.9 BLOOD TRANSFUSION
9.1 NON- INVASIVE PCO2 MONITORING
9.11 WHOLE BLOOD ANALYSIS
9.12 OTHERS
10 NORTH AMERICA POINT-OF-CARE TESTING (POCT) MARKET, BY PRESCRIPTION MODE
10.1 OVERVIEW
10.2 OTC TESTING
10.3 PRESCRIPTION-BASED TESTING
11 NORTH AMERICA POINT-OF-CARE-TESTING (POCT) MARKET, BY DISTRIBUTION CHANNEL
11.1 OVERVIEW
11.2 DIRECT TENDER
11.3 RETAIL SALES
11.4 ONLINE SALES
11.5 OTHERS
12 NORTH AMERICA POINT-OF-CARE TESTING (POCT) MARKET, BY END USER
12.1 OVERVIEW
12.2 HOSPITALS
12.2.1 PRIVATE
12.2.1.1 Tier 1
12.2.1.2 Tier 2
12.2.1.3 Tier 3
12.2.2 PUBLIC
12.2.2.1 Tier 1
12.2.2.2 Tier 2
12.2.2.3 Tier 3
12.3 HOME CARE
12.4 CLINICS
12.5 LABORATORIES
12.6 DIAGNOSTIC CENTERS
12.7 PATHOLOGY LABS
12.8 AMBULATORY SURGERY CENTERS
12.9 ELDERLY CARE CENTERS
12.1 OTHERS
13 NORTH AMERICA POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION
13.1 NORTH AMERICA
13.1.1 U.S.
13.1.2 CANADA
13.1.3 MEXICO
14 NORTH AMERICA POINT-OF-CARE TESTING (POCT) MARKET: COMPANY LANDSCAPE
14.1 COMPANY SHARE ANALYSIS: GLOBAL
15 SWOT ANALYSIS
16 COMPANY PROFILES
16.1 ABBOTT POINT OF CARE INC(ABBOTT)
16.1.1 COMPANY SNAPSHOT
16.1.2 REVENUE ANALYSIS
16.1.3 COMPANY SHARE ANALYSIS
16.1.4 PRODUCT PORTFOLIO
16.1.5 RECENT DEVELOPMENT
16.2 SINOCARE.
16.2.1 COMPANY SNAPSHOT
16.2.2 COMPANY SHARE ANALYSIS
16.2.3 PRODUCT PORTFOLIO
16.2.4 RECENT DEVELOPMENT
16.3 F. HOFFMANN-LA ROCHE LTD
16.3.1 COMPANY SNAPSHOT
16.3.2 REVENUE ANALYSIS
16.3.3 COMPANY SHARE ANALYSIS
16.3.4 PRODUCT PORTFOLIO
16.3.5 RECENT DEVELOPMENT
16.4 DANAHER
16.4.1 COMPANY SNAPSHOT
16.4.2 REVENUE ANALYSIS
16.4.3 COMPANY SHARE ANALYSIS
16.4.4 PRODUCT PORTFOLIO
16.4.5 RECENT DEVELOPMENT
16.5 HOLOGIC, INC
16.5.1 COMPANY SNAPSHOT
16.5.2 REVENUE ANALYSIS
16.5.3 COMPANY SHARE ANALYSIS
16.5.4 PRODUCT PORTFOLIO
16.5.5 RECENT DEVELOPMENT
16.6 ACCUBIOTECH CO., LTD.
16.6.1 COMPANY SNAPSHOT
16.6.2 PRODUCT PORTFOLIO
16.6.3 RECENT DEVELOPMENT
16.7 ABAXIS (ABAXIS IS A PART OF ZOETIS)
16.7.1 COMPANY SNAPSHOT
16.7.2 PRODUCT PORTFOLIO
16.7.3 RECENT DEVELOPMENT
16.8 AUTOBIO
16.8.1 COMPANY SNAPSHOT
16.8.2 REVENUE ANALYSIS
16.8.3 PRODUCT PORTFOLIO
16.8.4 RECENT DEVELOPMENT
16.9 BD VERITOR(BD)
16.9.1 COMPANY SNAPSHOT
16.9.2 REVENUE ANALYSIS
16.9.3 PRODUCT PORTFOLIO
16.9.4 RECENT DEVELOPMENT
16.1 BINX HEALTH
16.10.1 COMPANY SNAPSHOT
16.10.2 SOLUTION PORTFOLIO
16.10.3 RECENT DEVELOPMENT
16.11 BIOMERIEUX
16.11.1 COMPANY SNAPSHOT
16.11.2 REVENUE ANALYSIS
16.11.3 PRODUCT PORTFOLIO
16.11.4 RECENT DEVELOPMENT
16.12 BIO- RAD LABORATORIES, INC.
16.12.1 COMPANY SNAPSHOT
16.12.2 REVENUE ANALYSIS
16.12.3 PRODUCT PORTFOLIO
16.12.4 RECENT DEVELOPMENT
16.13 CHEMBIO DIAGNOSTICS, INC.
16.13.1 COMPANY SNAPSHOT
16.13.2 PRODUCT PORTFOLIO
16.13.3 RECENT DEVELOPMENT
16.14 EKF DIAGNOSTICS HOLDINGS PLC
16.14.1 COMPANY SNAPSHOT
16.14.2 REVENUE ANALYSIS
16.14.3 PRODUCT PORTFOLIO
16.14.4 RECENT DEVELOPMENT
16.15 GETEIN BIOTECH, INC.
16.15.1 COMPANY SNAPSHOT
16.15.2 REVENUE ANALYSIS
16.15.3 PRODUCT PORTFOLIO
16.15.4 RECENT DEVELOPMENT
16.16 LAMDAGEN CORPORATION
16.16.1 COMPANY SNAPSHOT
16.16.2 PRODUCT PORTFOLIO
16.16.3 RECENT DEVELOPMENT
16.17 MERIDIAN BIOSCIENCE
16.17.1 COMPANY SNAPSHOT
16.17.2 PRODUCT PORTFOLIO
16.17.3 RECENT DEVELOPMENT
16.18 NOVA BIOMEDICAL
16.18.1 COMPANY SNAPSHOT
16.18.2 PRODUCT PORTFOLIO
16.18.3 RECENT DEVELOPMENT
16.19 PFIZER INC.
16.19.1 COMPANY SNAPSHOT
16.19.2 PRODUCT PORTFOLIO
16.19.3 RECENT DEVELOPMENT
16.2 PTS DIAGNOSTICS
16.20.1 COMPANY SNAPSHOT
16.20.2 PRODUCT PORTFOLIO
16.20.3 RECENT DEVELOPMENT
16.21 QIAGEN
16.21.1 COMPANY SNAPSHOT
16.21.2 REVENUE ANALYSIS
16.21.3 PRODUCT PORTFOLIO
16.21.4 RECENT DEVELOPMENT
16.22 QUIDELORTHO CORPORATION
16.22.1 COMPANY SNAPSHOT
16.22.2 REVENUE ANALYSIS
16.22.3 PRODUCT PORTFOLIO
16.22.4 RECENT DEVELOPMENT
16.23 QUANTUMDX GROUP LTD.
16.23.1 COMPANY SNAPSHOT
16.23.2 PRODUCT PORTFOLIO
16.23.3 RECENT DEVELOPMENT
16.24 SEKISUI DIAGNOSTICS
16.24.1 COMPANY SNAPSHOT
16.24.2 PRODUCT PORTFOLIO
16.24.3 RECENT. DEVELOPMENT
16.25 SHENZHEN NEW INDUSTRY BIOMEDICAL ENGINEERING CO., LTD.
16.25.1 COMPANY SNAPSHOT
16.25.2 REVENUE ANALYSIS
16.25.3 PRODUCT PORTFOLIO
16.25.4 RECENT DEVELOPMENT
16.26 SIEMENS HEALTHINEERS AG
16.26.1 COMPANY SNAPSHOT
16.26.2 REVENUE ANALYSIS
16.26.3 PRODUCT PORTFOLIO
16.26.4 RECENT DEVELOPMENT
16.27 SIENCO, INC.
16.27.1 COMPANY SNAPSHOT
16.27.2 PRODUCT PORTFOLIO
16.27.3 RECENT UPDATES
16.28 SYSMEX CORPORATION
16.28.1 COMPANY SNAPSHOT
16.28.2 REVENUE ANALYSIS
16.28.3 PRODUCT PORTFOLIO
16.28.4 RECENT DEVELOPMENT
16.29 TRINITY BIOTECH
16.29.1 COMPANY SNAPSHOT
16.29.2 REVENUE ANALYSIS
16.29.3 PR.ODUCT PORTFOLIO
16.29.4 RECENT DEVELOPMENT
16.3 TRIVIDIA HEALTH, INC.
16.30.1 COMPANY SNAPSHOT
16.30.2 PRODUCT PORTFOLIO
16.30.3 RECENT UPDATES
16.31 THERMO FISHER SCIENTIFIC INC.
16.31.1 COMPANY SNAPSHOT
16.31.2 REVENUE ANALYSIS
16.31.3 PRODUCT PORTFOLIO
16.31.4 RECENT DEVELOPMENT
16.32 WERFEN
16.32.1 COMPANY SNAPSHOT
16.32.2 PRODUCT PORTFOLIO
16.32.3 RECENT DEVELOPMENT
16.33 WONDFO
16.33.1 COMPANY SNAPSHOT
16.33.2 REVENUE ANALYSIS
16.33.3 PRODUCT PORTFOLIO
16.33.4 RECENT DEVELOPMENT
16.34 XIAMEN BOSON BIOTECH CO., LTD.
16.34.1 COMPANY SNAPSHOT
16.34.2 PRODUCT PORTFOLIO
16.34.3 RECENT DEVELOPMENT
17 QUESTIONNAIRE
18 RELATED REPORTS
Lista de Tablas
TABLE 1 NORTH AMERICA POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 2 NORTH AMERICA POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 3 NORTH AMERICA POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 4 NORTH AMERICA GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD THOUSAND)
TABLE 5 NORTH AMERICA GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 6 NORTH AMERICA GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 7 NORTH AMERICA GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 8 NORTH AMERICA SELF-MONITORING OF BLOOD GLUCOSE (SMBG) DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 9 NORTH AMERICA SELF-MONITORING OF BLOOD GLUCOSE (SMBG) DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 10 NORTH AMERICA SELF-MONITORING OF BLOOD GLUCOSE (SMBG) DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 11 NORTH AMERICA INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD THOUSAND)
TABLE 12 NORTH AMERICA INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSANDS)
TABLE 13 NORTH AMERICA INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSANDS UNITS)
TABLE 14 NORTH AMERICA INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 15 NORTH AMERICA HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 16 NORTH AMERICA HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 17 NORTH AMERICA HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 18 NORTH AMERICA SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 19 NORTH AMERICA SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 20 NORTH AMERICA SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 21 NORTH AMERICA HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSANDS)
TABLE 22 NORTH AMERICA HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 23 NORTH AMERICA HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 24 NORTH AMERICA INFLUENZA TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 25 NORTH AMERICA TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 26 NORTH AMERICA TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 27 NORTH AMERICA TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 28 NORTH AMERICA MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 29 NORTH AMERICA MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 30 NORTH AMERICA MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 31 NORTH AMERICA CARDIOMETABOLIC MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD THOUSAND)
TABLE 32 NORTH AMERICA CARDIOMETABOLIC MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 33 NORTH AMERICA POC ANALYZER IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 34 NORTH AMERICA POC ANALYZER IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 35 NORTH AMERICA CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 36 NORTH AMERICA CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 37 NORTH AMERICA CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 38 NORTH AMERICA BLOOD GAS/ELECTROLYTE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 39 NORTH AMERICA BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 40 NORTH AMERICA BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 41 NORTH AMERICA BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 42 NORTH AMERICA BLOOD GAS/ELECTROLYTE TESTING INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 43 NORTH AMERICA PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 44 NORTH AMERICA PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 45 NORTH AMERICA PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 46 NORTH AMERICA BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSANDS)
TABLE 47 NORTH AMERICA BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 48 NORTH AMERICA BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 49 NORTH AMERICA HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 50 NORTH AMERICA HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 51 NORTH AMERICA HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 52 NORTH AMERICA ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 53 NORTH AMERICA ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 54 NORTH AMERICA ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 55 NORTH AMERICA PREGNANCY AND FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD THOUSAND)
TABLE 56 NORTH AMERICA PREGNANCY AND FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 57 NORTH AMERICA PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 58 NORTH AMERICA PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSANDS UNITS)
TABLE 59 NORTH AMERICA PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 60 NORTH AMERICA FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 61 NORTH AMERICA FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 62 NORTH AMERICA FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 63 NORTH AMERICA HAEMATOLOGY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD THOUSAND)
TABLE 64 NORTH AMERICA COAGULATION MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD THOUSANDS)
TABLE 65 NORTH AMERICA COAGULATION MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSANDS)
TABLE 66 NORTH AMERICA ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 67 NORTH AMERICA ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 68 NORTH AMERICA ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 69 NORTH AMERICA VISCOELASTIC COAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY METHOD, 2018-2033 (USD THOUSANDS)
TABLE 70 NORTH AMERICA DRUGS-OF-ABUSE (DOA) TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD THOUSAND)
TABLE 71 NORTH AMERICA DRUG-OF-ABUSE (DOA) TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 72 NORTH AMERICA DOA ANALYZERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSANDS)
TABLE 73 NORTH AMERICA DOA ANALYZERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 74 NORTH AMERICA DOA ANALYZERS MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 75 NORTH AMERICA RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 76 NORTH AMERICA RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 77 NORTH AMERICA RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 78 NORTH AMERICA URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD THOUSAND)
TABLE 79 NORTH AMERICA URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 80 NORTH AMERICA URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 81 NORTH AMERICA URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 82 NORTH AMERICA CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD THOUSAND)
TABLE 83 NORTH AMERICA CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 84 NORTH AMERICA CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSANDS UNITS)
TABLE 85 NORTH AMERICA CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 86 NORTH AMERICA INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSANDS)
TABLE 87 NORTH AMERICA INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 88 NORTH AMERICA INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 89 NORTH AMERICA TUMOR/CANCER MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD THOUSAND)
TABLE 90 NORTH AMERICA FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD THOUSAND)
TABLE 91 NORTH AMERICA FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 92 NORTH AMERICA FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSANDS UNITS)
TABLE 93 NORTH AMERICA FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 94 NORTH AMERICA OTHERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD THOUSAND)
TABLE 95 NORTH AMERICA POINT-OF-CARE-TESTING (POCT) MARKET, BY PLATFORM, 2018-2033 (USD THOUSAND)
TABLE 96 NORTH AMERICA LATERAL FLOW ASSAYS/IMMUNOCHROMATOGRAPHY TESTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD THOUSANDS)
TABLE 97 NORTH AMERICA IMMUNOASSAYS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD THOUSANDS)
TABLE 98 NORTH AMERICA DIPSTICKS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD THOUSANDS)
TABLE 99 NORTH AMERICA MOLECULAR DIAGNOSTICS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD THOUSANDS)
TABLE 100 NORTH AMERICA CLINICAL CHEMISTRY ASSAYS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD THOUSAND)
TABLE 101 NORTH AMERICA MICROFLUIDICS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD THOUSANDS)
TABLE 102 NORTH AMERICA HEMATOLOGY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD THOUSANDS)
TABLE 103 NORTH AMERICA OTHERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD THOUSANDS)
TABLE 104 NORTH AMERICA POINT-OF-CARE-TESTING (POCT) MARKET, BY APPLICATION, 2018-2033 (USD THOUSAND)
TABLE 105 NORTH AMERICA BLOOD GLUCOSE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD THOUSAND)
TABLE 106 NORTH AMERICA INFECTIOUS DISEASES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD THOUSAND)
TABLE 107 NORTH AMERICA INFECTIOUS DISEASES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 108 NORTH AMERICA VITAL SIGN MONITORING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD THOUSAND)
TABLE 109 NORTH AMERICA CARDIAC MONITORING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD THOUSAND)
TABLE 110 NORTH AMERICA COAGULATION IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD THOUSAND)
TABLE 111 NORTH AMERICA HAEMATOLOGY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD THOUSAND)
TABLE 112 NORTH AMERICA NON-INVASIVE SPO2 MONITORING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD THOUSAND)
TABLE 113 NORTH AMERICA BLOOD TRANSFUSION IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD THOUSAND)
TABLE 114 NORTH AMERICA NON-INVASIVE PCO2 MONITORING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD THOUSAND)
TABLE 115 NORTH AMERICA WHOLE BLOOD ANALYSIS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD THOUSAND)
TABLE 116 NORTH AMERICA OTHERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD THOUSAND)
TABLE 117 NORTH AMERICA POINT-OF-CARE-TESTING (POCT) MARKET, BY PRESCRIPTION MODE, 2018-2033 (USD THOUSANDS)
TABLE 118 NORTH AMERICA OTC TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD THOUSAND)
TABLE 119 NORTH AMERICA PRESCRIPTION-BASED TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD THOUSANDS)
TABLE 120 NORTH AMERICA POINT-OF-CARE-TESTING (POCT) MARKET, BY DISTRIBUTION CHANNEL, 2018-2033 (USD MILLION)
TABLE 121 NORTH AMERICA DIRECT TENDER IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD MILLIONS)
TABLE 122 NORTH AMERICA RETAIL SALES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD MILLIONS)
TABLE 123 NORTH AMERICA ONLINE SALES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD MILLIONS)
TABLE 124 NORTH AMERICA OTHERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2033 (USD MILLIONS)
TABLE 125 NORTH AMERICA POINT-OF-CARE-TESTING (POCT) MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 126 NORTH AMERICA HOSPITALS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2032 (USD THOUSANDS)
TABLE 127 NORTH AMERICA HOSPITALS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2032 (USD THOUSANDS)
TABLE 128 NORTH AMERICA PRIVATE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY LEVEL, 2018-2032 (USD THOUSANDS)
TABLE 129 NORTH AMERICA PUBLIC IN POINT-OF-CARE-TESTING (POCT) MARKET, BY LEVEL, 2018-2032 (USD THOUSANDS)
TABLE 130 NORTH AMERICA HOME CARE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 131 NORTH AMERICA CLINICS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 132 NORTH AMERICA LABORATORIES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 133 NORTH AMERICA DIAGNOSTIC CENTERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 134 NORTH AMERICA PATHOLOGY LABS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 135 NORTH AMERICA AMBULATORY SURGERY CENTERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 136 NORTH AMERICA ELDERLY CARE CENTERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 137 NORTH AMERICA OTHERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 138 NORTH AMERICA POINT-OF-CARE-TESTING (POCT) MARKET, BY COUNTRY, 2018-2033 (USD THOUSAND)
TABLE 139 NORTH AMERICA POINT-OF-CARE-TESTING (POCT) MARKET, BY COUNTRY, 2018-2033 (USD THOUSAND)
TABLE 140 NORTH AMERICA
TABLE 141 NORTH AMERICA POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 142 NORTH AMERICA POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 143 NORTH AMERICA POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 144 NORTH AMERICA GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 145 NORTH AMERICA GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 146 NORTH AMERICA GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 147 NORTH AMERICA SELF-MONITORING OF BLOOD GLUCOSE (SMBG) DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 148 NORTH AMERICA SELF-MONITORING OF BLOOD GLUCOSE (SMBG) DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 149 NORTH AMERICA SELF-MONITORING OF BLOOD GLUCOSE (SMBG) DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 150 NORTH AMERICA INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 151 NORTH AMERICA INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 152 NORTH AMERICA INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 153 NORTH AMERICA HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 154 NORTH AMERICA HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 155 NORTH AMERICA HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 156 NORTH AMERICA SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 157 NORTH AMERICA SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 158 NORTH AMERICA SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 159 NORTH AMERICA HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 160 NORTH AMERICA HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 161 NORTH AMERICA HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 162 NORTH AMERICA INFLUENZA TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 163 NORTH AMERICA TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 164 NORTH AMERICA TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 165 NORTH AMERICA TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 166 NORTH AMERICA MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 167 NORTH AMERICA MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 168 NORTH AMERICA MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 169 NORTH AMERICA CARDIOMETABOLIC MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 170 NORTH AMERICA POC ANALYZER IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 171 NORTH AMERICA POC ANALYZER IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 172 NORTH AMERICA CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 173 NORTH AMERICA CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 174 NORTH AMERICA CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 175 NORTH AMERICA BLOOD GAS/ELECTROLYTE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 176 NORTH AMERICA BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 177 NORTH AMERICA BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 178 NORTH AMERICA BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 179 NORTH AMERICA BLOOD GAS/ELECTROLYTE TESTING INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 180 NORTH AMERICA PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 181 NORTH AMERICA PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 182 NORTH AMERICA PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 183 NORTH AMERICA BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 184 NORTH AMERICA BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 185 NORTH AMERICA BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 186 NORTH AMERICA HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 187 NORTH AMERICA HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 188 NORTH AMERICA HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 189 NORTH AMERICA ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 190 NORTH AMERICA ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 191 NORTH AMERICA ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 192 NORTH AMERICA PREGNANCY AND FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 193 NORTH AMERICA PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 194 NORTH AMERICA PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 195 NORTH AMERICA PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 196 NORTH AMERICA FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 197 NORTH AMERICA FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 198 NORTH AMERICA FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 199 NORTH AMERICA COAGULATION MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 200 NORTH AMERICA ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 201 NORTH AMERICA ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 202 NORTH AMERICA ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 203 NORTH AMERICA VISCOELASTIC COAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY METHOD, 2018-2033 (USD THOUSAND)
TABLE 204 NORTH AMERICA DRUG-OF-ABUSE (DOA) TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 205 NORTH AMERICA DOA ANALYZERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 206 NORTH AMERICA DOA ANALYZERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 207 NORTH AMERICA DOA ANALYZERS MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 208 NORTH AMERICA RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 209 NORTH AMERICA RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 210 NORTH AMERICA RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 211 NORTH AMERICA URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 212 NORTH AMERICA URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 213 NORTH AMERICA URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 214 NORTH AMERICA CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 215 NORTH AMERICA CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 216 NORTH AMERICA CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 217 NORTH AMERICA INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 218 NORTH AMERICA INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 219 NORTH AMERICA INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 220 NORTH AMERICA FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 221 NORTH AMERICA FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 222 NORTH AMERICA FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 223 NORTH AMERICA POINT-OF-CARE-TESTING (POCT) MARKET, BY PLATFORM, 2018-2033 (USD THOUSAND)
TABLE 224 NORTH AMERICA POINT-OF-CARE-TESTING (POCT) MARKET, BY APPLICATION, 2018-2033 (USD THOUSAND)
TABLE 225 NORTH AMERICA INFECTIOUS DISEASES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 226 NORTH AMERICA POINT-OF-CARE-TESTING (POCT) MARKET, BY PRESCRIPTION MODE, 2018-2033 (USD THOUSAND)
TABLE 227 NORTH AMERICA POINT-OF-CARE-TESTING (POCT) MARKET, BY END USER, 2018-2033 (USD THOUSAND)
TABLE 228 NORTH AMERICA HOSPITALS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 229 NORTH AMERICA PRIVATE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY LEVEL, 2018-2033 (USD THOUSAND)
TABLE 230 NORTH AMERICA PUBLIC IN POINT-OF-CARE-TESTING (POCT) MARKET, BY LEVEL, 2018-2033 (USD THOUSAND)
TABLE 231 NORTH AMERICA POINT-OF-CARE-TESTING (POCT) MARKET, BY DISTRIBUTION CHANNEL, 2018-2033 (USD THOUSAND)
TABLE 232 U.S. POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 233 U.S. POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 234 U.S. POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 235 U.S. GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 236 U.S. GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 237 U.S. GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 238 U.S. SELF-MONITORING OF BLOOD GLUCOSE (SMBG) DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 239 U.S. SELF-MONITORING OF BLOOD GLUCOSE (SMBG) DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 240 U.S. SELF-MONITORING OF BLOOD GLUCOSE (SMBG) DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 241 U.S. INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 242 U.S. INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 243 U.S. INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 244 U.S. HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 245 U.S. HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 246 U.S. HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 247 U.S. SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 248 U.S. SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 249 U.S. SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 250 U.S. HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 251 U.S. HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 252 U.S. HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 253 U.S. INFLUENZA TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 254 U.S. TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 255 U.S. TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 256 U.S. TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 257 U.S. MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 258 U.S. MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 259 U.S. MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 260 U.S. CARDIOMETABOLIC MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 261 U.S. POC ANALYZER IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 262 U.S. POC ANALYZER IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 263 U.S. CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 264 U.S. CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 265 U.S. CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 266 U.S. BLOOD GAS/ELECTROLYTE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 267 U.S. BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 268 U.S. BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 269 U.S. BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 270 U.S. BLOOD GAS/ELECTROLYTE TESTING INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 271 U.S. PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 272 U.S. PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 273 U.S. PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 274 U.S. BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 275 U.S. BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 276 U.S. BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 277 U.S. HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 278 U.S. HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 279 U.S. HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 280 U.S. ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 281 U.S. ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 282 U.S. ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 283 U.S. PREGNANCY AND FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 284 U.S. PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 285 U.S. PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 286 U.S. PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 287 U.S. FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 288 U.S. FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 289 U.S. FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 290 U.S. COAGULATION MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 291 U.S. ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 292 U.S. ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 293 U.S. ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 294 U.S. VISCOELASTIC COAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY METHOD, 2018-2033 (USD THOUSAND)
TABLE 295 U.S. DRUG-OF-ABUSE (DOA) TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 296 U.S. DOA ANALYZERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 297 U.S. DOA ANALYZERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 298 U.S. DOA ANALYZERS MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 299 U.S. RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 300 U.S. RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 301 U.S. RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 302 U.S. URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 303 U.S. URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 304 U.S. URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 305 U.S. CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 306 U.S. CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 307 U.S. CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 308 U.S. INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 309 U.S. INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 310 U.S. INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 311 U.S. FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 312 U.S. FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 313 U.S. FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 314 U.S. POINT-OF-CARE-TESTING (POCT) MARKET, BY PLATFORM, 2018-2033 (USD THOUSAND)
TABLE 315 U.S. POINT-OF-CARE-TESTING (POCT) MARKET, BY APPLICATION, 2018-2033 (USD THOUSAND)
TABLE 316 U.S. INFECTIOUS DISEASES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 317 U.S. POINT-OF-CARE-TESTING (POCT) MARKET, BY PRESCRIPTION MODE, 2018-2033 (USD THOUSAND)
TABLE 318 U.S. POINT-OF-CARE-TESTING (POCT) MARKET, BY END USER, 2018-2033 (USD THOUSAND)
TABLE 319 U.S. HOSPITALS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 320 U.S. PRIVATE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY LEVEL, 2018-2033 (USD THOUSAND)
TABLE 321 U.S. PUBLIC IN POINT-OF-CARE-TESTING (POCT) MARKET, BY LEVEL, 2018-2033 (USD THOUSAND)
TABLE 322 U.S. POINT-OF-CARE-TESTING (POCT) MARKET, BY DISTRIBUTION CHANNEL, 2018-2033 (USD THOUSAND)
TABLE 323 CANADA POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 324 CANADA POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 325 CANADA POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 326 CANADA GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 327 CANADA GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 328 CANADA GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 329 CANADA SELF-MONITORING OF BLOOD GLUCOSE (SMBG) DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 330 CANADA SELF-MONITORING OF BLOOD GLUCOSE (SMBG) DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 331 CANADA SELF-MONITORING OF BLOOD GLUCOSE (SMBG) DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 332 CANADA INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 333 CANADA INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 334 CANADA INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 335 CANADA HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 336 CANADA HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 337 CANADA HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 338 CANADA SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 339 CANADA SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 340 CANADA SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 341 CANADA HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 342 CANADA HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 343 CANADA HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 344 CANADA INFLUENZA TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 345 CANADA TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 346 CANADA TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 347 CANADA TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 348 CANADA MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 349 CANADA MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 350 CANADA MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 351 CANADA CARDIOMETABOLIC MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 352 CANADA POC ANALYZER IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 353 CANADA POC ANALYZER IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 354 CANADA CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 355 CANADA CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 356 CANADA CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 357 CANADA BLOOD GAS/ELECTROLYTE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 358 CANADA BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 359 CANADA BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 360 CANADA BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 361 CANADA BLOOD GAS/ELECTROLYTE TESTING INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 362 CANADA PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 363 CANADA PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 364 CANADA PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 365 CANADA BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 366 CANADA BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 367 CANADA BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 368 CANADA HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 369 CANADA HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 370 CANADA HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 371 CANADA ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 372 CANADA ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 373 CANADA ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 374 CANADA PREGNANCY AND FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 375 CANADA PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 376 CANADA PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 377 CANADA PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 378 CANADA FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 379 CANADA FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 380 CANADA FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 381 CANADA COAGULATION MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 382 CANADA ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 383 CANADA ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 384 CANADA ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 385 CANADA VISCOELASTIC COAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY METHOD, 2018-2033 (USD THOUSAND)
TABLE 386 CANADA DRUG-OF-ABUSE (DOA) TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 387 CANADA DOA ANALYZERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 388 CANADA DOA ANALYZERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 389 CANADA DOA ANALYZERS MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 390 CANADA RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 391 CANADA RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 392 CANADA RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 393 CANADA URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 394 CANADA URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 395 CANADA URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 396 CANADA CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 397 CANADA CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 398 CANADA CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 399 CANADA INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 400 CANADA INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 401 CANADA INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 402 CANADA FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 403 CANADA FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 404 CANADA FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 405 CANADA POINT-OF-CARE-TESTING (POCT) MARKET, BY PLATFORM, 2018-2033 (USD THOUSAND)
TABLE 406 CANADA POINT-OF-CARE-TESTING (POCT) MARKET, BY APPLICATION, 2018-2033 (USD THOUSAND)
TABLE 407 CANADA INFECTIOUS DISEASES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 408 CANADA POINT-OF-CARE-TESTING (POCT) MARKET, BY PRESCRIPTION MODE, 2018-2033 (USD THOUSAND)
TABLE 409 CANADA POINT-OF-CARE-TESTING (POCT) MARKET, BY END USER, 2018-2033 (USD THOUSAND)
TABLE 410 CANADA HOSPITALS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 411 CANADA PRIVATE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY LEVEL, 2018-2033 (USD THOUSAND)
TABLE 412 CANADA PUBLIC IN POINT-OF-CARE-TESTING (POCT) MARKET, BY LEVEL, 2018-2033 (USD THOUSAND)
TABLE 413 CANADA POINT-OF-CARE-TESTING (POCT) MARKET, BY DISTRIBUTION CHANNEL, 2018-2033 (USD THOUSAND)
TABLE 414 MEXICO POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 415 MEXICO POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 416 MEXICO POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 417 MEXICO GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 418 MEXICO GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 419 MEXICO GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 420 MEXICO SELF-MONITORING OF BLOOD GLUCOSE (SMBG) DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 421 MEXICO SELF-MONITORING OF BLOOD GLUCOSE (SMBG) DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 422 MEXICO SELF-MONITORING OF BLOOD GLUCOSE (SMBG) DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 423 MEXICO INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 424 MEXICO INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 425 MEXICO INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 426 MEXICO HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 427 MEXICO HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 428 MEXICO HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 429 MEXICO SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 430 MEXICO SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 431 MEXICO SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 432 MEXICO HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 433 MEXICO HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 434 MEXICO HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 435 MEXICO INFLUENZA TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 436 MEXICO TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 437 MEXICO TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 438 MEXICO TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 439 MEXICO MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 440 MEXICO MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 441 MEXICO MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 442 MEXICO CARDIOMETABOLIC MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 443 MEXICO POC ANALYZER IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 444 MEXICO POC ANALYZER IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 445 MEXICO CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 446 MEXICO CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 447 MEXICO CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 448 MEXICO BLOOD GAS/ELECTROLYTE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 449 MEXICO BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 450 MEXICO BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 451 MEXICO BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 452 MEXICO BLOOD GAS/ELECTROLYTE TESTING INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 453 MEXICO PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 454 MEXICO PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 455 MEXICO PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 456 MEXICO BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 457 MEXICO BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 458 MEXICO BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 459 MEXICO HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 460 MEXICO HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 461 MEXICO HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 462 MEXICO ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 463 MEXICO ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 464 MEXICO ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 465 MEXICO PREGNANCY AND FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 466 MEXICO PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 467 MEXICO PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 468 MEXICO PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 469 MEXICO FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 470 MEXICO FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 471 MEXICO FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 472 MEXICO COAGULATION MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 473 MEXICO ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 474 MEXICO ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 475 MEXICO ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 476 MEXICO VISCOELASTIC COAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY METHOD, 2018-2033 (USD THOUSAND)
TABLE 477 MEXICO DRUG-OF-ABUSE (DOA) TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 478 MEXICO DOA ANALYZERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 479 MEXICO DOA ANALYZERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 480 MEXICO DOA ANALYZERS MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 481 MEXICO RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 482 MEXICO RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 483 MEXICO RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 484 MEXICO URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 485 MEXICO URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 486 MEXICO URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 487 MEXICO CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 488 MEXICO CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 489 MEXICO CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 490 MEXICO INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 491 MEXICO INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 492 MEXICO INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 493 MEXICO FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (USD THOUSAND)
TABLE 494 MEXICO FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (VOLUME IN THOUSAND UNITS)
TABLE 495 MEXICO FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2033 (ASP IN USD/UNITS)
TABLE 496 MEXICO POINT-OF-CARE-TESTING (POCT) MARKET, BY PLATFORM, 2018-2033 (USD THOUSAND)
TABLE 497 MEXICO POINT-OF-CARE-TESTING (POCT) MARKET, BY APPLICATION, 2018-2033 (USD THOUSAND)
TABLE 498 MEXICO INFECTIOUS DISEASES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 499 MEXICO POINT-OF-CARE-TESTING (POCT) MARKET, BY PRESCRIPTION MODE, 2018-2033 (USD THOUSAND)
TABLE 500 MEXICO POINT-OF-CARE-TESTING (POCT) MARKET, BY END USER, 2018-2033 (USD THOUSAND)
TABLE 501 MEXICO HOSPITALS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 502 MEXICO PRIVATE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY LEVEL, 2018-2033 (USD THOUSAND)
TABLE 503 MEXICO PUBLIC IN POINT-OF-CARE-TESTING (POCT) MARKET, BY LEVEL, 2018-2033 (USD THOUSAND)
TABLE 504 MEXICO POINT-OF-CARE-TESTING (POCT) MARKET, BY DISTRIBUTION CHANNEL, 2018-2033 (USD THOUSAND)
Lista de figuras
FIGURE 1 NORTH AMERICA POINT-OF-CARE-TESTING (POCT) MARKET: SEGMENTATION
FIGURE 2 NORTH AMERICA POINT-OF-CARE-TESTING (POCT) MARKET: DATA TRIANGULATION
FIGURE 3 NORTH AMERICA POINT-OF-CARE-TESTING (POCT) MARKET: DROC ANALYSIS
FIGURE 4 NORTH AMERICA POINT-OF-CARE-TESTING (POCT) MARKET: REGIONAL MARKET ANALYSIS
FIGURE 5 NORTH AMERICA POINT-OF-CARE-TESTING (POCT) MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 NORTH AMERICA POINT-OF-CARE-TESTING (POCT) MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 NORTH AMERICA POINT-OF-CARE-TESTING (POCT) MARKET: DBMR MARKET POSITION GRID
FIGURE 8 NORTH AMERICA POINT-OF-CARE-TESTING (POCT) MARKET: MARKET END USER COVERAGE GRID
FIGURE 9 NORTH AMERICA POINT-OF-CARE-TESTING (POCT) MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 NORTH AMERICA POINT-OF-CARE-TESTING (POCT) MARKET: SEGMENTATION
FIGURE 11 NORTH AMERICA POINT-OF-CARE-TESTING (POCT) MARKET EXECUTIVE SUMMARY
FIGURE 12 STRATEGIC DECISIONS
FIGURE 13 RISING USE OF POC TESTING IN HEALTHCARE FACILITIES IS EXPECTED TO DRIVE THE GROWTH OF NORTH AMERICA POINT-OF-CARE-TESTING (POCT) MARKET FROM 2026 TO 2033
FIGURE 14 THE GLUCOSE MONITORING PRODUCTS SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE NORTH AMERICA POINT-OF-CARE-TESTING (POCT) MARKET IN 2026 - 2032
FIGURE 15 DRCO ANALYSIS
FIGURE 16 NORTH AMERICA POINT-OF-CARE-TESTING (POCT) MARKET: BY PRODUCT TYPE, 2025
FIGURE 17 NORTH AMERICA POINT-OF-CARE-TESTING (POCT) MARKET: BY PRODUCT TYPE, 2026-2033 (USD THOUSAND)
FIGURE 18 NORTH AMERICA POINT-OF-CARE-TESTING (POCT) MARKET: BY PRODUCT TYPE, CAGR (2026-2033)
FIGURE 19 NORTH AMERICA POINT-OF-CARE-TESTING (POCT) MARKET: BY PRODUCT TYPE, LIFELINE CURVE
FIGURE 20 NORTH AMERICA POINT-OF-CARE TESTING (POCT) MARKET: BY PLATFORM, 2025
FIGURE 21 NORTH AMERICA POINT-OF-CARE TESTING (POCT) MARKET: BY PLATFORM, 2026 TO 2033 (USD THOUSAND)
FIGURE 22 NORTH AMERICA POINT-OF-CARE TESTING (POCT) MARKET: BY PLATFORM, CAGR (2026- 2033)
FIGURE 23 NORTH AMERICA POINT-OF-CARE TESTING (POCT) MARKET: BY PLATFORM, LIFELINE CURVE
FIGURE 24 NORTH AMERICA POINT-OF-CARE TESTING (POCT) MARKET: BY APPLICATION, 2025
FIGURE 25 NORTH AMERICA POINT-OF-CARE TESTING (POCT) MARKET: BY APPLICATION, 2026 TO 2033 (USD THOUSAND)
FIGURE 26 NORTH AMERICA POINT-OF-CARE TESTING (POCT) MARKET: BY APPLICATION, CAGR (2026- 2033)
FIGURE 27 NORTH AMERICA POINT-OF-CARE TESTING (POCT) MARKET: BY APPLICATION, LIFELINE CURVE
FIGURE 28 NORTH AMERICA POINT-OF-CARE TESTING (POCT) MARKET: BY PRESCRIPTION MODE, 2025
FIGURE 29 NORTH AMERICA POINT-OF-CARE TESTING (POCT) MARKET: BY PRESCRIPTION MODE, 2026 TO 2033 (USD THOUSAND)
FIGURE 30 NORTH AMERICA POINT-OF-CARE TESTING (POCT) MARKET: BY PRESCRIPTION MODE, CAGR (2026- 2033)
FIGURE 31 NORTH AMERICA POINT-OF-CARE TESTING (POCT) MARKET: BY PRESCRIPTION MODE, LIFELINE CURVE
FIGURE 32 NORTH AMERICA POINT-OF-CARE TESTING (POCT) MARKET: BY DISTRIBUTION CHANNEL, 2025
FIGURE 33 NORTH AMERICA POINT-OF-CARE TESTING (POCT) MARKET: BY DISTRIBUTION CHANNEL, 2026 TO 2033 (USD THOUSAND)
FIGURE 34 NORTH AMERICA POINT-OF-CARE TESTING (POCT) MARKET: BY DISTRIBUTION CHANNEL, CAGR (2026- 2033)
FIGURE 35 NORTH AMERICA POINT-OF-CARE TESTING (POCT) MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 36 NORTH AMERICA POINT-OF-CARE TESTING (POCT) MARKET: BY END USER, 2025
FIGURE 37 NORTH AMERICA POINT-OF-CARE TESTING (POCT) MARKET: BY END USER, 2026 TO 2033 (USD THOUSAND)
FIGURE 38 NORTH AMERICA POINT-OF-CARE TESTING (POCT) MARKET: BY END USER, CAGR (2026- 2033)
FIGURE 39 NORTH AMERICA POINT-OF-CARE TESTING (POCT) MARKET: BY END USER, LIFELINE CURVE
FIGURE 40 NORTH AMERICA POINT-OF-CARE-TESTING (POCT) MARKET: SNAPSHOT
FIGURE 41 NORTH AMERICA POINT-OF-CARE TESTING (POCT) MARKET: COMPANY SHARE 2025 (%)
Metodología de investigación
La recopilación de datos y el análisis del año base se realizan utilizando módulos de recopilación de datos con muestras de gran tamaño. La etapa incluye la obtención de información de mercado o datos relacionados a través de varias fuentes y estrategias. Incluye el examen y la planificación de todos los datos adquiridos del pasado con antelación. Asimismo, abarca el examen de las inconsistencias de información observadas en diferentes fuentes de información. Los datos de mercado se analizan y estiman utilizando modelos estadísticos y coherentes de mercado. Además, el análisis de la participación de mercado y el análisis de tendencias clave son los principales factores de éxito en el informe de mercado. Para obtener más información, solicite una llamada de un analista o envíe su consulta.
La metodología de investigación clave utilizada por el equipo de investigación de DBMR es la triangulación de datos, que implica la extracción de datos, el análisis del impacto de las variables de datos en el mercado y la validación primaria (experto en la industria). Los modelos de datos incluyen cuadrícula de posicionamiento de proveedores, análisis de línea de tiempo de mercado, descripción general y guía del mercado, cuadrícula de posicionamiento de la empresa, análisis de patentes, análisis de precios, análisis de participación de mercado de la empresa, estándares de medición, análisis global versus regional y de participación de proveedores. Para obtener más información sobre la metodología de investigación, envíe una consulta para hablar con nuestros expertos de la industria.
Personalización disponible
Data Bridge Market Research es líder en investigación formativa avanzada. Nos enorgullecemos de brindar servicios a nuestros clientes existentes y nuevos con datos y análisis que coinciden y se adaptan a sus objetivos. El informe se puede personalizar para incluir análisis de tendencias de precios de marcas objetivo, comprensión del mercado de países adicionales (solicite la lista de países), datos de resultados de ensayos clínicos, revisión de literatura, análisis de mercado renovado y base de productos. El análisis de mercado de competidores objetivo se puede analizar desde análisis basados en tecnología hasta estrategias de cartera de mercado. Podemos agregar tantos competidores sobre los que necesite datos en el formato y estilo de datos que esté buscando. Nuestro equipo de analistas también puede proporcionarle datos en archivos de Excel sin procesar, tablas dinámicas (libro de datos) o puede ayudarlo a crear presentaciones a partir de los conjuntos de datos disponibles en el informe.