North America Surgical Sutures Market
Tamaño del mercado en miles de millones de dólares
Tasa de crecimiento anual compuesta (CAGR) :
% 
 USD
15.18 Billion
USD
26.45 Billion
2024
2032
USD
15.18 Billion
USD
26.45 Billion
2024
2032
| 2025 –2032 | |
| USD 15.18 Billion | |
| USD 26.45 Billion | |
|
|
|
|
North America Surgical Sutures Market, By Product (Suture Threads and Automated Suturing Devices), Type (Multifilament Sutures and Monofilament Sutures), Application (Cardiovascular Surgery, General Surgery, Gynaecological Surgery, Orthopaedic Surgeries, Ophthalmic Surgery, Cosmetic and Plastic Surgery, and Other Applications), End-User (Hospitals, Ambulatory Surgical Centres (ASC), Clinics and Physician Offices), Country (U.S., Canada and Mexico)_ Industry Trends and Forecast to 2032
Surgical Sutures Market Size
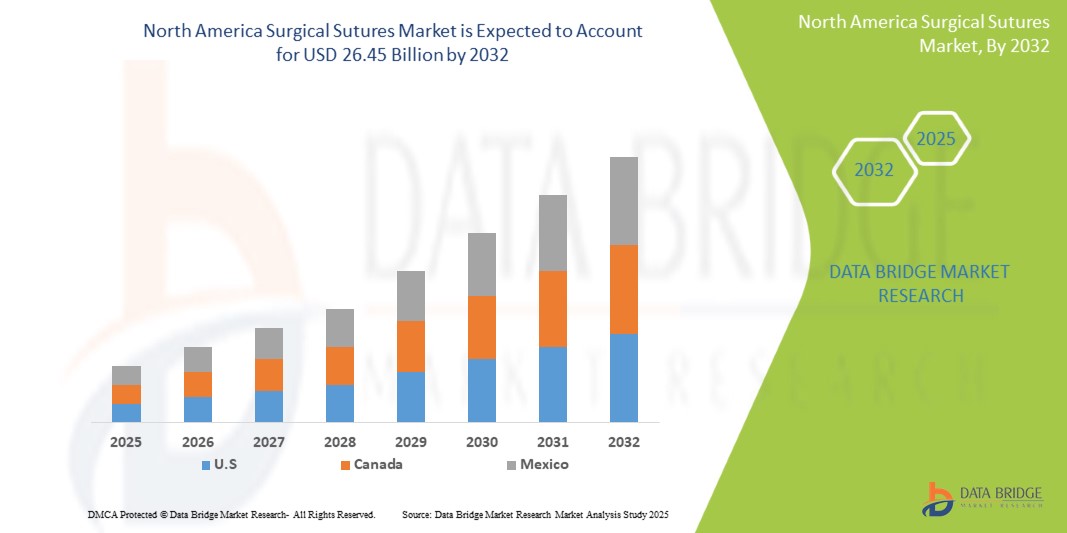
- The North America Surgical Sutures Market size was valued atUSD 15.18 Billion in 2024and is expected to reachUSD 26.45 Billion by 2032, at aCAGR of 6.55%during the forecast period
- This growth is driven by factors such as the rising number of surgical procedures, increasing incidence of chronic wounds, and technological advancements in Surgical Sutures.
Surgical Sutures Market Analysis
- Active wound care products play a critical role in enhancing the wound healing process by supporting cellular activity and tissue regeneration, particularly in chronic and non-healing wounds
- The demand for these products is driven by the increasing prevalence of chronic wounds, growing geriatric population, and rising awareness about advanced wound management solutions
- U.S. is expected to dominate the Active and Surgical Sutures market due to its strong healthcare infrastructure, high healthcare expenditure, and increasing adoption of innovative wound care therapies
- U.S. is also projected to be the fastest-growing market during the forecast period due to the surge in diabetic foot ulcers, supportive reimbursement policies, and growing investments in wound care R&D
- The Advanced Dressings segment is expected to lead the market with a significant share due to their effectiveness in moisture retention, infection prevention, and acceleration of the healing process in complex wounds
Report Scope andSurgical Sutures Market Segmentation
|
Attributes |
Surgical Sutures KeyMarket Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include import export analysis, production capacity overview, production consumption analysis, price trend analysis, climate change scenario, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Surgical Sutures Market Trends
“Rising Prevalence of Chronic and Surgical Wounds”
- The increasing incidence of chronic wounds, such as diabetic foot ulcers and pressure ulcers, is driving demand for effective Surgical Sutures devices to accelerate healing, minimize infection risk, and reduce hospital stays, especially among the aging population.
- Surgical procedures are rising due to a growing elderly population and lifestyle-related diseases. Post-surgical wound management is critical, and advanced Surgical Sutures devices are essential to support quick recovery and minimize complications like infections and dehiscence.
- Patients suffering from obesity or compromised immune systems face more complications in wound healing. This necessitates innovative Surgical Sutures solutions that offer superior sealing, minimal trauma, and improved outcomes, thereby fueling the adoption of advanced Surgical Sutures.
Surgical Sutures Market Dynamics
Driver
Technological Advancements in Surgical Sutures Techniques
- Emerging technologies such as absorbable staples, bioengineered adhesives, and antimicrobial sutures provide efficient, less invasive wound Surgical Sutures options. These innovations enhance clinical outcomes and patient comfort, encouraging wider use in both hospitals and outpatient settings.
- The integration of Surgical Sutures with digital monitoring tools enables real-time assessment of healing progress. This improves treatment strategies, reduces readmissions, and supports better decision-making among healthcare professionals, promoting adoption across North America.
- Minimally invasive surgical procedures are increasingly popular, necessitating equally advanced Surgical Sutures. These tools allow quicker recovery times, reduced scarring, and better cosmetic results, meeting rising patient and provider expectations for high-quality care.
Opportunity
“Rising Ambulatory Surgical Centers (ASCs) and Homecare Trends”
- With the shift toward cost-effective healthcare, Ambulatory Surgical Centers are on the rise. These facilities favor wound Surgical Sutures solutions that are quick to apply, easy to monitor, and adaptable to same-day procedures, offering growth potential for manufacturers.
- The expansion of home healthcare services allows for increased use of user-friendly Surgical Sutures. Devices like skin adhesives and adhesive strips, which require minimal medical supervision, are in demand among homecare patients across North America.
- Remote patient monitoring combined with easy-to-use Surgical Sutures technologies creates an opportunity for manufacturers to develop smart, self-applicable Surgical Sutures systems. These innovations align with the growing preference for decentralized and patient-centric healthcare delivery models.
Restraint/Challenge
“Regulatory Hurdles and Reimbursement Constraints”
- Strict regulatory pathways for approval of Surgical Sutures in the U.S. and Canada can delay market entry. Developers face rigorous testing and documentation requirements, increasing time-to-market and development costs.
- Reimbursement limitations for newer wound Surgical Sutures technologies restrict hospital and clinic adoption. Without adequate insurance coverage, healthcare providers may be reluctant to switch from traditional methods, hindering widespread deployment of innovative solutions.
- Navigating varying state-level reimbursement rules in the U.S. adds complexity for manufacturers. Inconsistent policies between regions impact market penetration and create operational challenges, especially for small and mid-sized device developers.
Surgical Sutures Market Scope
The market is segmented on the basis By Device, Application, Type of Wound, End User.
|
Segmentation |
Sub-Segmentation |
|
By Product |
|
|
Type |
|
|
Application |
|
|
End-User |
|
In 2025, theAdhesives is projected to dominate the market with a largest share in application segment
The Adhesives segment is expected to dominate the Surgical Sutures market with the largest share of 48.38% in 2024 due to increasing demand for non-invasive, easy-to-use wound Surgical Sutures solutions. As a critical component in both surgical and trauma care, advancements in adhesive strength, flexibility, and biocompatibility have significantly improved clinical outcomes and patient comfort. The rising volume of surgical procedures, growing preference for minimally invasive techniques, and increasing healthcare awareness further contribute to the segment’s market dominance.
TheStaples is expected to account for the largest share during the forecast period in technology market
In 2025, the Staples segment is expected to dominate the Surgical Sutures market with the largest market share of 48.38% due to their efficiency, speed of application, and effectiveness in closing large or complex wounds. As a vital component in surgical wound management, advancements in stapler design, material safety, and precision delivery systems have improved procedural outcomes and reduced operative time. The increasing number of surgical interventions, rising demand for reliable Surgical Sutures methods, and growing adoption of technology-driven surgical tools further contribute to the segment’s market dominance.
Surgical Sutures Market Regional Analysis
“U.S Holds the Largest Share and highest CAGR in the Surgical Sutures Market”
- U.S. dominates and highest CAGR the Surgical Sutures market, driven by advanced healthcare infrastructure, high volume of surgical procedures, and strong presence of key market players
- The U.S. holds a significant share due to increased demand for minimally invasive wound Surgical Sutures solutions, rising incidence of chronic wounds, and growing preference for faster recovery options
- The availability of well-established reimbursement policies and growing investments in surgical innovation by leading medical device companies further strengthen the market
- In addition, the increasing shift toward outpatient surgeries, integration of smart wound care technologies, and the rising elderly population are fueling market expansion across the region
Surgical Sutures Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- Johnson & Johnson Services, Inc. (U.S.)
- Medtronic (Ireland)
- 3M (U.S.)
- Smith+Nephew (U.K.)
- B. Braun SE (Germany)
- Stryker (U.S.)
- Baxter (U.S.)
- Boston Scientific Corporation (U.S.)
- Frankenman International Ltd. (China)
- CooperSurgical Inc. (U.S.)
- Intuitive Surgical (U.S.)
- MANI, INC. (Japan)
- Artivion, Inc. (U.S.)
- CP Medical (Riverpoint Medical) (U.S.)
- CONMED Corporation (U.S.)
- Genesis Medtech (Singapore)
- Cardinal Health, Inc. (U.S.)
- Essity AB (Sweden)
- Medline Industries, LP (U.S.)
Latest Developments in North America Surgical Sutures Market
- In June 2021, Ethicon Plus sutures were the first to be approved for use in the NHS by NICE Medical Technologies Guidance as they have been demonstrated to lower the possibility of surgical site infections (SSIs) by over 30%.
- In November 2020, Trubarb, a knotless tissue Surgical Sutures device launched by Healthium, is an efficacious triangular end stopper that removes the need for knotting as compared to a normal suture.
- In March 2020, LiquiBand Plus, developed by Advanced Medical Solutions Limited, was approved by the FDA to heal readily approximated skin margins of wounds after surgical incisions.
- In February 2020, Wego-Stainless Steel suture was approved by the FDA for applications such as abdominal wound Surgical Sutures, hernia repair, and sternal Surgical Sutures by Foosin Medical Supplies Inc., Ltd
SKU-
Obtenga acceso en línea al informe sobre la primera nube de inteligencia de mercado del mundo
- Panel de análisis de datos interactivo
- Panel de análisis de empresas para oportunidades con alto potencial de crecimiento
- Acceso de analista de investigación para personalización y consultas
- Análisis de la competencia con panel interactivo
- Últimas noticias, actualizaciones y análisis de tendencias
- Aproveche el poder del análisis de referencia para un seguimiento integral de la competencia
Metodología de investigación
La recopilación de datos y el análisis del año base se realizan utilizando módulos de recopilación de datos con muestras de gran tamaño. La etapa incluye la obtención de información de mercado o datos relacionados a través de varias fuentes y estrategias. Incluye el examen y la planificación de todos los datos adquiridos del pasado con antelación. Asimismo, abarca el examen de las inconsistencias de información observadas en diferentes fuentes de información. Los datos de mercado se analizan y estiman utilizando modelos estadísticos y coherentes de mercado. Además, el análisis de la participación de mercado y el análisis de tendencias clave son los principales factores de éxito en el informe de mercado. Para obtener más información, solicite una llamada de un analista o envíe su consulta.
La metodología de investigación clave utilizada por el equipo de investigación de DBMR es la triangulación de datos, que implica la extracción de datos, el análisis del impacto de las variables de datos en el mercado y la validación primaria (experto en la industria). Los modelos de datos incluyen cuadrícula de posicionamiento de proveedores, análisis de línea de tiempo de mercado, descripción general y guía del mercado, cuadrícula de posicionamiento de la empresa, análisis de patentes, análisis de precios, análisis de participación de mercado de la empresa, estándares de medición, análisis global versus regional y de participación de proveedores. Para obtener más información sobre la metodología de investigación, envíe una consulta para hablar con nuestros expertos de la industria.
Personalización disponible
Data Bridge Market Research es líder en investigación formativa avanzada. Nos enorgullecemos de brindar servicios a nuestros clientes existentes y nuevos con datos y análisis que coinciden y se adaptan a sus objetivos. El informe se puede personalizar para incluir análisis de tendencias de precios de marcas objetivo, comprensión del mercado de países adicionales (solicite la lista de países), datos de resultados de ensayos clínicos, revisión de literatura, análisis de mercado renovado y base de productos. El análisis de mercado de competidores objetivo se puede analizar desde análisis basados en tecnología hasta estrategias de cartera de mercado. Podemos agregar tantos competidores sobre los que necesite datos en el formato y estilo de datos que esté buscando. Nuestro equipo de analistas también puede proporcionarle datos en archivos de Excel sin procesar, tablas dinámicas (libro de datos) o puede ayudarlo a crear presentaciones a partir de los conjuntos de datos disponibles en el informe.