Europe Breast Biopsy Devices Market
Taille du marché en milliards USD
TCAC :
% 
 USD
1.25 Billion
USD
2.01 Billion
2024
2032
USD
1.25 Billion
USD
2.01 Billion
2024
2032
| 2025 –2032 | |
| USD 1.25 Billion | |
| USD 2.01 Billion | |
|
|
|
|
Segmentation du marché européen des dispositifs de biopsie mammaire, par produit (aiguilles, tables, fils, systèmes de guidage, pistolets à biopsie, etc.), type de technique (biopsie par aspiration à l'aiguille fine, biopsie à l'aiguille, marqueurs de biopsie, biopsie à l'aiguille guidée par IRM, biopsie chirurgicale, localisation par fil et biopsie du ganglion sentinelle), technologie de guidage (biopsie guidée par échographie fine, résonance magnétique guidée par mammographie, biopsie guidée par scanner et autres biopsies mammaires guidées par imagerie), utilisateur final (hôpitaux, centres de chirurgie ambulatoire, centres de diagnostic, etc.) - Tendances et prévisions du secteur jusqu'en 2032
Taille du marché des dispositifs de biopsie mammaire
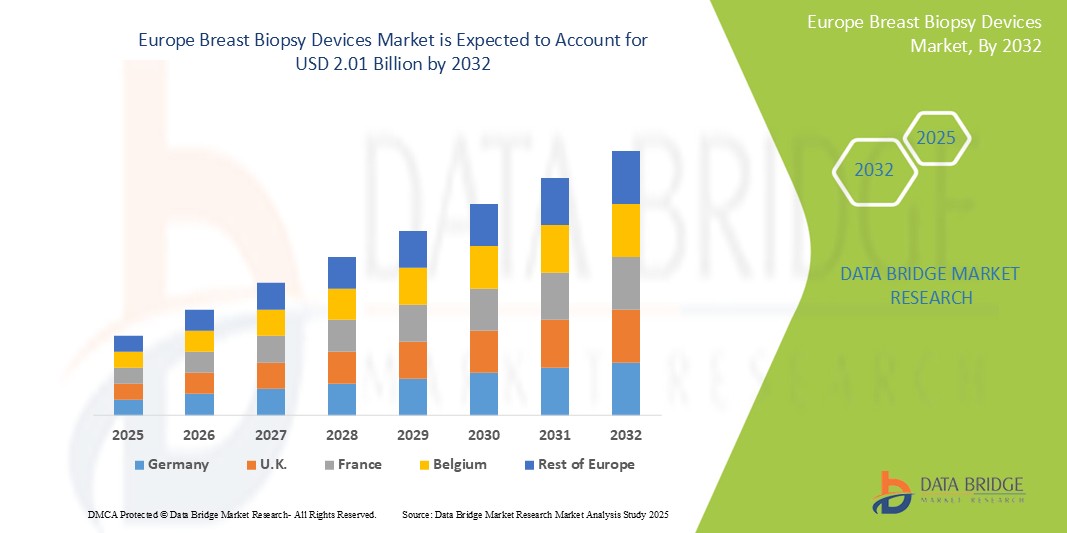
- La taille du marché européen des dispositifs de biopsie mammaire était évaluée à 1,25 milliard USD en 2024 et devrait atteindre 2,01 milliards USD d'ici 2032 , à un TCAC de 7,20 % au cours de la période de prévision.
- La croissance du marché est largement alimentée par l’incidence croissante du cancer du sein en Europe, ainsi que par la sensibilisation et l’importance croissantes accordées au diagnostic précoce, ce qui stimule la demande de dispositifs de biopsie mammaire avancés dans les milieux hospitaliers et ambulatoires.
- De plus, les progrès technologiques dans les techniques de biopsie mini-invasive, associés à l'augmentation des dépenses de santé et à la préférence des patients pour des méthodes de diagnostic précises et plus sûres, font des dispositifs de biopsie mammaire un élément essentiel du diagnostic moderne du cancer. Ces facteurs convergents accélèrent l'adoption de ces solutions, stimulant ainsi considérablement la croissance du secteur.
Analyse du marché des dispositifs de biopsie mammaire
- Les dispositifs de biopsie mammaire, y compris les technologies avancées telles que les aiguilles de biopsie, les tables, les fils et les systèmes de guidage, deviennent des outils de plus en plus essentiels dans la détection et le diagnostic précoces du cancer du sein dans les hôpitaux, les centres de diagnostic et les établissements de chirurgie ambulatoire à travers l'Europe, en raison de leur précision, de leur nature mini-invasive et de l'amélioration des résultats pour les patients.
- La demande croissante de dispositifs de biopsie mammaire est principalement alimentée par la prévalence croissante du cancer du sein, des politiques de remboursement robustes et l'adoption croissante de procédures de diagnostic guidées par l'image et mini-invasives en Allemagne et en France.
- L'Allemagne domine le marché des dispositifs de biopsie mammaire en Europe avec la plus grande part de revenus de 43,8 % en 2025, caractérisée par une infrastructure de soins de santé avancée, des niveaux de sensibilisation élevés, des dépenses de santé substantielles et l'adoption précoce de techniques de biopsie technologiquement avancées, notamment les biopsies guidées par IRM et stéréotaxiques.
- The Germany is expected to be the fastest-growing country in the Europe Breast Biopsy Devices market during the forecast period, driven by increasing patient preference for outpatient procedures, expansion of breast cancer screening programs, and a strong presence of key market players continuously launching innovative biopsy systems.
- Core Needle Biopsy is expected to dominate the Europe Breast Biopsy Devices market with a market share of 43.2% in 2025, driven by its clinical accuracy, cost-effectiveness, and growing utilization as a standard procedure for diagnosing palpable and non-palpable breast lesions.
Report Scope and Breast Biopsy Devices Market Segmentation
|
Attributes |
Breast Biopsy Devices Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
Europe
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Breast Biopsy Devices Market Trends
“Enhanced Accuracy Through AI and Imaging Integration”
- A significant and accelerating trend in the Europe Breast Biopsy Devices market is the deepening integration with artificial intelligence (AI) and advanced imaging technologies such as MRI, ultrasound, and 3D mammography. This fusion of technologies is significantly enhancing diagnostic precision, procedural efficiency, and patient outcomes.
- For instance, AI-assisted breast biopsy platforms are increasingly being adopted to improve lesion targeting, automate needle positioning, and support radiologists in interpreting complex imaging results more effectively. These intelligent systems help reduce human error and standardize biopsy procedures across clinical settings.
- AI integration in Breast Biopsy Devices enables features such as real-time imaging analytics, predictive diagnostics, and automated workflow support. For example, certain platforms utilize machine learning algorithms to detect and prioritize suspicious lesions, while also offering enhanced guidance for core needle and MRI-guided biopsies.
- The seamless integration of Breast Biopsy Devices with hospital information systems (HIS), PACS, and radiology workflow tools facilitates centralized access to imaging data, biopsy history, and patient records—enabling radiologists and surgeons to make faster, data-driven decisions and streamline interdepartmental collaboration.
- This trend toward more intelligent, data-integrated, and image-guided biopsy systems is fundamentally reshaping the standard of care in breast diagnostics. Consequently, companies such as Hologic and Siemens Healthineers are developing AI-enabled biopsy platforms with automated targeting, enhanced needle guidance, and integration with cloud-based diagnostic software.
- The demand for Breast Biopsy Devices that offer seamless AI and imaging system integration is growing rapidly across hospitals, diagnostic centers, and ambulatory surgical units, as clinicians increasingly prioritize precision medicine, workflow optimization, and minimally invasive diagnostic solutions.
Breast Biopsy Devices Market Dynamics
Driver
“Growing Need Due to Rising Breast Cancer Incidence and Advancements in Diagnostic Technology”
- The increasing prevalence of breast cancer across Europe, coupled with rising awareness about early detection and diagnosis, is a significant driver for the heightened demand for Breast Biopsy Devices
- For instance, according to the American Cancer Society, approximately 1 in 8 women in the Germany will develop invasive breast cancer over the course of her lifetime. This high incidence rate emphasizes the need for accurate and minimally invasive diagnostic tools, propelling market growth
- As healthcare providers focus on early-stage diagnosis to improve treatment outcomes, Breast Biopsy Devices offer advanced capabilities such as image-guided targeting, real-time tissue sampling, and reduced procedure times—providing a substantial improvement over traditional open surgical biopsy methods
- Furthermore, the integration of digital imaging technologies and AI-based guidance systems with biopsy devices is enhancing diagnostic precision and workflow efficiency, making them essential tools in modern breast cancer diagnostics.
- The preference for minimally invasive techniques, growing adoption of outpatient procedures, and increasing investments by hospitals and diagnostic centers in upgrading their biopsy capabilities are key factors propelling the adoption of Breast Biopsy Devices across the Europen healthcare ecosystem
Restraint/Challenge
“Concerns Regarding Procedural Complexity and High Equipment Costs”
- Concerns surrounding the procedural complexity and the high cost of advanced breast biopsy systems present a significant challenge to widespread market adoption, particularly among smaller healthcare facilities and rural clinics.
- For instance, MRI-guided breast biopsies, while highly accurate, require specialized infrastructure, trained personnel, and costly imaging systems—factors that can limit their availability to larger urban hospitals and academic centers.
- The need for continuous training of radiologists and interventional specialists to operate sophisticated biopsy technologies also adds to operational burden, especially in regions facing a shortage of skilled healthcare professionals.
- Additionally, high initial costs associated with installing stereotactic or vacuum-assisted biopsy systems can be a deterrent for budget-constrained institutions. This can limit market penetration despite clinical demand, especially in underfunded or underserved areas.
- While ongoing technological advancements and strategic partnerships are helping lower production costs and improve ease of use, addressing these challenges through government funding, insurance coverage expansion, and simplified device interfaces will be crucial for unlocking full market potential across Europe.
Breast Biopsy Devices Market Scope
The market is segmented on the basis product, technique type, guidance technology, and end user.
- By Product
On the basis of product, the breast biopsy devices market is segmented into biopsy needles, biopsy tables, biopsy wires, guidance systems, needle-based biopsy guns, and others. The biopsy needles segment dominates the largest market revenue share of 38.5% in 2025, driven by their critical role in both core needle and fine needle aspiration procedures. These needles are essential for extracting tissue samples with minimal invasiveness, and their compatibility with various imaging techniques enhances diagnostic accuracy. The segment benefits from continual innovations such as vacuum-assisted designs and improved tip geometries that facilitate precision targeting of lesions.
The guidance systems segment is anticipated to witness the fastest growth rate of 19.4% from 2025 to 2032, fueled by the rising preference for image-guided biopsies and the demand for real-time accuracy. These systems—integrated with ultrasound, MRI, and stereotactic platforms—help ensure precise localization of suspicious tissues, reduce sampling errors, and shorten procedure times, thereby improving clinical outcomes.
- By Technique Type
On the basis of technique type, the market is segmented into fine needle aspiration biopsy, core needle biopsy, biopsy markers, MRI-guided core needle biopsy, surgical biopsy, wire localization, and sentinel node biopsy. The core needle biopsy segment held the largest market revenue share in 2025, owing to its widespread use as the gold standard for diagnosing breast abnormalities. Its minimally invasive nature, ability to extract sufficient tissue, and lower risk compared to surgical procedures make it the preferred choice among clinicians.
The MRI-guided core needle biopsy segment is expected to witness the fastest CAGR from 2025 to 2032, driven by its superior imaging capabilities for detecting non-palpable and complex lesions. As MRI becomes more integrated into routine breast cancer diagnostics, its guidance in biopsies ensures enhanced accuracy in lesion targeting, especially in high-risk patients.
- By Guidance Technology
On the basis of guidance technology, the market is segmented into ultrasound-guided, mammography-guided, magnetic resonance-guided, CT-guided biopsy, and other image-guided breast biopsy. The ultrasound-guided biopsy segment accounted for the largest market revenue share in 2025, attributed to its real-time imaging capability, cost-effectiveness, and widespread availability. It is particularly effective for guiding needle placement in cystic or solid palpable lesions and is commonly used in both hospitals and ambulatory settings.
The magnetic resonance-guided biopsy segment is projected to witness the fastest CAGR from 2025 to 2032, as MR imaging is increasingly adopted for patients with dense breast tissue or inconclusive mammograms. The precision and sensitivity of MRI contribute to more accurate diagnoses, especially in high-risk screening programs.
- By End User
On the basis of end user, the market is segmented into hospitals, ambulatory surgical centers, diagnostic centers, and others.
The hospital segment holds the largest market revenue share in 2025, driven by the concentration of advanced diagnostic technologies, skilled professionals, and integrated imaging and biopsy workflows in these settings. Hospitals serve as referral centers for complex cases requiring MRI or stereotactic biopsies and play a leading role in early detection programs. The ambulatory surgical centers segment is expected to witness the fastest growth from 2025 to 2032, owing to the increasing shift toward outpatient procedures and the cost-efficiency of these settings. ASCs offer faster turnaround, reduced patient wait times, and minimally invasive biopsy procedures under local anesthesia, making them attractive for both patients and healthcare providers.
Breast Biopsy Devices Market Regional Analysis
- Germany dominates the Breast Biopsy Devices market with the largest revenue share of 43.8% in 2024, driven by the increasing prevalence of breast cancer, advanced healthcare infrastructure, and early adoption of minimally invasive diagnostic technologies.
- The country’s favorable reimbursement landscape, extensive breast cancer screening programs (such as mammography mandates under the Affordable Care Act), and rising awareness about early detection significantly contribute to the dominance of the Germany market.
- The adoption of image-guided biopsy procedures in ambulatory settings and hospitals is also on the rise due to their higher accuracy and reduced patient trauma, thereby fueling overall market growth in the Germany
France Breast Biopsy Devices Market Insight
The France Breast Biopsy Devices market is projected to expand at a substantial CAGR throughout the forecast period, driven by the increasing incidence of breast cancer, rising awareness about early detection, and advancements in minimally invasive biopsy techniques. Government initiatives supporting cancer screening programs and the growing adoption of image-guided biopsy procedures further propel market growth. Additionally, technological innovations enhancing accuracy and patient comfort, alongside the presence of well-established healthcare infrastructure, are contributing to the increasing demand for breast biopsy devices in France.
U.K. Breast Biopsy Devices Market Insight
The U.K. Breast Biopsy Devices market is anticipated to grow at a noteworthy CAGR during the forecast period, fueled by improvements in healthcare infrastructure, urbanization, and increasing government focus on cancer care. The launch of national programs for cancer detection and awareness campaigns (such as "Octubre Rosa") are encouraging women to undergo routine breast screening and diagnostic procedures, thereby driving demand for biopsy devices. While access to advanced biopsy systems is currently limited to urban and private healthcare facilities, ongoing public-private partnerships and foreign investments in medical technologies are expected to broaden device accessibility across regions. Increased training of radiologists and pathologists, along with the incorporation of ultrasound and mammography-guided biopsy solutions, is anticipated to further accelerate the adoption of breast biopsy devices in U.K.
Breast Biopsy Devices Market Share
The Breast Biopsy Devices industry is primarily led by well-established companies, including:
- SurgicEye GmbH (Germany)
- Hologic, Inc. (U.S.)
- BD (Becton, Dickinson and Company) (U.S.)
- Stryker Corporation (U.S.)
- Siemens Healthineers (Germany)
- Devicor Medical Products (U.S.)
- Elesta S.p.A. (Italy)
- Esaote S.p.A. (Italy)
- BIRADS GmbH (Germany)
- Scion Medical Technologies (U.K.)
- Biopsafe Ltd. (Finland)
- Surgiris (France)
- I.M.D. Generators S.r.l. (Italy)
- Medcom GmbH (Germany)
- TBS Group (Italy)
- Micrima Ltd. (U.K.)
- SuperSonic Imagine (France)
Latest Developments in Europe Breast Biopsy Devices Market
- In April 2024, Hologic, Inc., a global leader in women’s health, announced the Germany FDA clearance of its new Brevera Breast Biopsy System with CorLumina imaging technology. This all-in-one system streamlines the biopsy process by combining tissue acquisition, real-time imaging, and verification in a single step, reducing procedure time and enhancing patient comfort. The development underscores Hologic’s ongoing commitment to innovation in image-guided breast biopsy solutions and improving workflow efficiency in clinical settings
- In March 2024, BD (Becton, Dickinson and Company) launched the BD UltraCore Biopsy Device in Europe, targeting enhanced precision for core needle biopsy procedures. The device features a dual-spring system that improves tissue capture while minimizing patient trauma. The launch aligns with the rising demand for minimally invasive biopsy tools and supports BD’s expansion in the high-growth breast diagnostics segment
- In February 2024, Stryker Corporation announced a strategic partnership with multiple ambulatory surgical centers (ASCs) across the Germany to deploy its advanced breast biopsy navigation software, designed to assist surgeons in more accurately locating and sampling suspicious lesions. This partnership reflects the growing role of software-enabled biopsy guidance in improving diagnostic accuracy and supporting outpatient breast cancer diagnostics
- In December 2023, Devicor Medical Products (a Leica Biosystems company) received Health France approval for its MammoMARK Biopsy Site Marker, an advanced tissue marking solution used post-biopsy for site localization in follow-up treatments. The launch is expected to improve accuracy in surgical planning and reinforces Devicor’s leadership in post-biopsy care and tissue marking technologies in the Europen region
SKU-
Accédez en ligne au rapport sur le premier cloud mondial de veille économique
- Tableau de bord d'analyse de données interactif
- Tableau de bord d'analyse d'entreprise pour les opportunités à fort potentiel de croissance
- Accès d'analyste de recherche pour la personnalisation et les requêtes
- Analyse de la concurrence avec tableau de bord interactif
- Dernières actualités, mises à jour et analyse des tendances
- Exploitez la puissance de l'analyse comparative pour un suivi complet de la concurrence
Méthodologie de recherche
La collecte de données et l'analyse de l'année de base sont effectuées à l'aide de modules de collecte de données avec des échantillons de grande taille. L'étape consiste à obtenir des informations sur le marché ou des données connexes via diverses sources et stratégies. Elle comprend l'examen et la planification à l'avance de toutes les données acquises dans le passé. Elle englobe également l'examen des incohérences d'informations observées dans différentes sources d'informations. Les données de marché sont analysées et estimées à l'aide de modèles statistiques et cohérents de marché. De plus, l'analyse des parts de marché et l'analyse des tendances clés sont les principaux facteurs de succès du rapport de marché. Pour en savoir plus, veuillez demander un appel d'analyste ou déposer votre demande.
La méthodologie de recherche clé utilisée par l'équipe de recherche DBMR est la triangulation des données qui implique l'exploration de données, l'analyse de l'impact des variables de données sur le marché et la validation primaire (expert du secteur). Les modèles de données incluent la grille de positionnement des fournisseurs, l'analyse de la chronologie du marché, l'aperçu et le guide du marché, la grille de positionnement des entreprises, l'analyse des brevets, l'analyse des prix, l'analyse des parts de marché des entreprises, les normes de mesure, l'analyse globale par rapport à l'analyse régionale et des parts des fournisseurs. Pour en savoir plus sur la méthodologie de recherche, envoyez une demande pour parler à nos experts du secteur.
Personnalisation disponible
Data Bridge Market Research est un leader de la recherche formative avancée. Nous sommes fiers de fournir à nos clients existants et nouveaux des données et des analyses qui correspondent à leurs objectifs. Le rapport peut être personnalisé pour inclure une analyse des tendances des prix des marques cibles, une compréhension du marché pour d'autres pays (demandez la liste des pays), des données sur les résultats des essais cliniques, une revue de la littérature, une analyse du marché des produits remis à neuf et de la base de produits. L'analyse du marché des concurrents cibles peut être analysée à partir d'une analyse basée sur la technologie jusqu'à des stratégies de portefeuille de marché. Nous pouvons ajouter autant de concurrents que vous le souhaitez, dans le format et le style de données que vous recherchez. Notre équipe d'analystes peut également vous fournir des données sous forme de fichiers Excel bruts, de tableaux croisés dynamiques (Fact book) ou peut vous aider à créer des présentations à partir des ensembles de données disponibles dans le rapport.