Europe Postpartum Hemorrhage Treatment Devices Market
Taille du marché en milliards USD
TCAC :
% 
 USD
274.95 Million
USD
397.15 Million
2024
2032
USD
274.95 Million
USD
397.15 Million
2024
2032
| 2025 –2032 | |
| USD 274.95 Million | |
| USD 397.15 Million | |
|
|
|
|
Marché européen des dispositifs de traitement des hémorragies post-partum, type (tamponnade par ballonnet utérin, système d'injection prérempli Uniject, vêtement antichoc non pneumatique, dispositifs de contrôle des hémorragies induites par le vide, etc.), pathologie (hémorragie post-partum majeure (plus de 1 000 ml), hémorragie post-partum mineure (500 à 1 000 ml), hémorragie post-partum massive (2 000 ml ou plus) et hémorragie post-partum secondaire), type de patiente (HPP primaire et secondaire), utilisateur final (hôpitaux, maternités, cliniques spécialisées, établissements de soins à domicile, etc.), canal de distribution (appel d'offres direct, vente au détail, etc.) - Tendances et prévisions du secteur jusqu'en 2032
Taille du marché des dispositifs de traitement des hémorragies post-partum
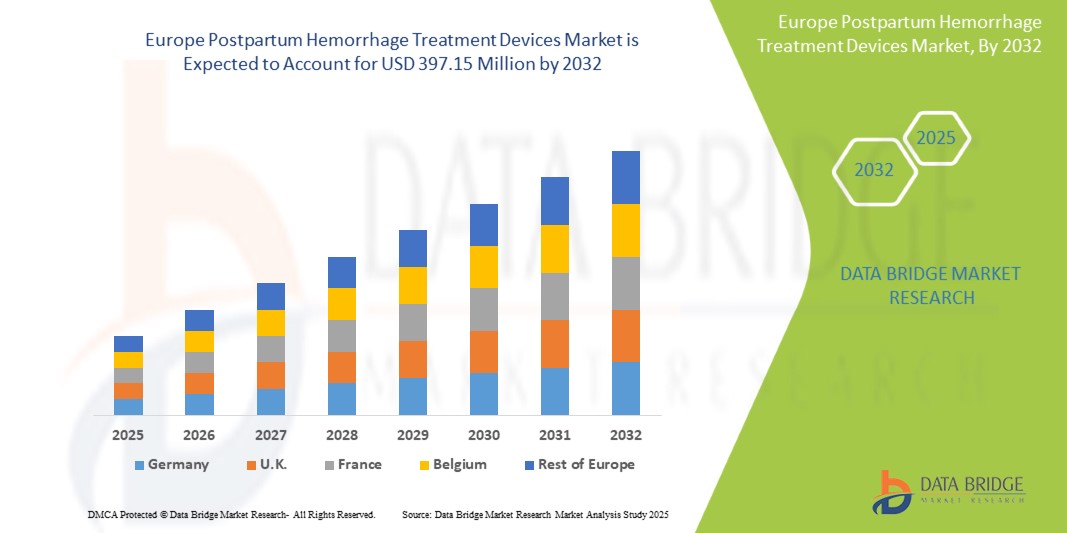
- Le marché européen des dispositifs de traitement des hémorragies post-partum était évalué à 274,95 millions USD en 2024 et devrait atteindre 397,15 millions USD d'ici 2032.
- Au cours de la période de prévision de 2025 à 2032, le marché devrait croître à un TCAC de 4,7 %, principalement en raison de l'incidence croissante des hémorragies post-partum.
- Les principaux moteurs du marché des dispositifs de traitement des hémorragies post-partum comprennent l’incidence croissante des hémorragies post-partum, la sensibilisation croissante aux traitements efficaces et les progrès technologiques.
Analyse des dispositifs de traitement des hémorragies post-partum
- L'incidence croissante des hémorragies post-partum, due à des facteurs tels que l'augmentation des taux d'accouchements par césarienne et des complications pendant l'accouchement, a entraîné une demande croissante de dispositifs de traitement efficaces.
- Les innovations en matière de technologie médicale, notamment les nouveaux dispositifs de tamponnement utérin, les agents hémostatiques et les options chirurgicales mini-invasives, contribuent à l’amélioration des résultats pour les patients et stimulent la croissance du marché.
- Par exemple, le marché des dispositifs de traitement de l'HPP varie géographiquement, avec une croissance significative observée dans les régions en développement en raison de l'augmentation des investissements dans les soins de santé et de l'accent mis sur la santé maternelle.
- Par conséquent, le marché des dispositifs de traitement de l'HPP présente une forte disparité géographique, les régions en développement connaissant une croissance significative en raison de l'augmentation des investissements dans les soins de santé et d'une approche ciblée visant à améliorer la santé maternelle.
Portée du rapport et segmentation des dispositifs de traitement des hémorragies post-partum
|
Attributs |
Dispositifs de traitement des hémorragies post-partum : informations clés sur le marché |
|
Segments couverts |
|
|
Pays couverts |
Europe
|
|
Acteurs clés du marché |
|
|
Opportunités de marché |
|
|
Ensembles d'informations de données à valeur ajoutée |
Outre les informations sur les scénarios de marché tels que la valeur marchande, le taux de croissance, la segmentation, la couverture géographique et les principaux acteurs, les rapports de marché organisés par Data Bridge Market Research comprennent également une analyse des importations et des exportations, un aperçu de la capacité de production, une analyse de la consommation de production, une analyse des tendances des prix, un scénario de changement climatique, une analyse de la chaîne d'approvisionnement, une analyse de la chaîne de valeur, un aperçu des matières premières/consommables, des critères de sélection des fournisseurs, une analyse PESTLE, une analyse Porter et un cadre réglementaire. |
Tendances du marché des dispositifs de traitement des hémorragies post-partum
« Adoption accrue des dispositifs de tamponnement utérin par ballonnet »
- Les dispositifs de tamponnement utérin par ballonnet sont conçus pour contrôler l'HPP en exerçant une pression directe sur la paroi utérine, favorisant ainsi l'hémostase. Leur efficacité en milieu clinique a été largement documentée, ce qui a renforcé la confiance des professionnels de santé dans leur utilisation en première intention pour la prise en charge de l'HPP, favorisant ainsi leur adoption.
- Par exemple, des efforts concertés ont été déployés au sein de la communauté médicale pour améliorer la formation et l'éducation à la prise en charge de l'HPP. Cela comprend une formation spécifique à l'utilisation des techniques de tamponnement utérin par ballonnet. La sensibilisation accrue des professionnels de santé à l'importance d'une intervention rapide a conduit à une acceptation et une utilisation plus larges de ces dispositifs, tant dans les pays développés que dans les pays en développement.
- Les dispositifs de tamponnement par ballonnet utérin sont de plus en plus intégrés dans les protocoles complets de soins maternels, en particulier dans les contextes obstétricaux d'urgence.
- Cette intégration s'appuie sur les recommandations d'organisations telles que l'OMS et diverses initiatives de santé maternelle, qui encouragent des pratiques fondées sur des données probantes pour prévenir et gérer efficacement l'HPP. L'harmonisation de ces dispositifs avec les recommandations cliniques facilite leur adoption généralisée dans les hôpitaux et les maisons de naissance.
Conducteurs
« Augmentation de l'incidence des hémorragies post-partum »
- L'incidence croissante de l'hémorragie du post-partum (HPP) a créé un besoin urgent de dispositifs de traitement efficaces sur le marché mondial. Une meilleure sensibilisation aux problèmes de santé maternelle et une meilleure identification des populations à risque ont entraîné une forte demande de solutions innovantes.
- Alors que les systèmes de santé accordent la priorité à la sécurité maternelle, l'adoption de dispositifs médicaux avancés visant à prévenir ou à gérer l'HPP est en hausse, notamment les dispositifs de compression utérine et les agents hémostatiques
Par exemple,
- En mai 2020, selon le NCBI, l'hémorragie du post-partum (HPP) était la principale cause de mortalité maternelle dans le monde. Aux États-Unis, l'HPP a augmenté de 26 %. Cette augmentation alarmante des cas souligne l'importance cruciale de la lutte contre l'HPP et encourage l'augmentation des investissements dans des solutions et technologies thérapeutiques innovantes visant à prévenir et à prendre en charge cette affection potentiellement mortelle.
- En août 2024, l'Organisation mondiale de la Santé a déclaré que l'hémorragie du post-partum (HPP), généralement définie comme une perte de sang de 500 ml ou plus dans les 24 heures suivant l'accouchement, était la principale cause de mortalité maternelle dans le monde. Elle touche des millions de femmes chaque année et représente plus de 20 % des décès maternels signalés dans le monde.
Opportunités
« Programmes de formation et d'éducation pour une utilisation appropriée des dispositifs de traitement de l'HPP »
- L’élaboration de programmes complets de formation et d’éducation pour l’utilisation appropriée des dispositifs de traitement de l’HPP (hémorragie post-partum) représente une opportunité précieuse pour renforcer les services de santé maternelle.
- Ces programmes peuvent être adaptés aux professionnels de la santé à différents niveaux, des agents de santé communautaires aux sages-femmes qualifiées et au personnel hospitalier, garantissant que tout le personnel impliqué dans les soins maternels dispose des connaissances nécessaires et de l'expérience pratique.
Contraintes/Défis
« Impact environnemental et problèmes d'élimination des dispositifs PPH à usage unique »
- Les dispositifs à usage unique pour l'hémorragie du post-partum (HPP), tels que les tamponnements par ballonnet utérin et les systèmes d'aspiration, génèrent d'importants déchets médicaux, ce qui pose des problèmes environnementaux et d'élimination, notamment dans les milieux à faibles ressources. Sans infrastructure adéquate de gestion des déchets, les dispositifs mis au rebut peuvent s'accumuler dans les décharges ou les décharges à ciel ouvert, libérant ainsi dans l'environnement des matières nocives comme le plastique et des résidus biologiques dangereux.
- Une élimination inappropriée augmente également le risque de récupération ou de réutilisation d'équipements contaminés dans des situations désespérées, augmentant ainsi le risque d'infections comme l'hépatite ou la septicémie chez les patients et le personnel soignant. Cette double menace, à savoir la dégradation de l'environnement et les risques pour la santé publique, souligne la nécessité de solutions de conception et d'élimination durables pour le développement des dispositifs d'HPP.
Par exemple,
- En mars 2022, le MDPI a souligné que l'utilisation croissante de dispositifs d'hémorragie post-partum à usage unique s'ajoute au volume croissant de déchets hospitaliers, dont une grande partie est dangereuse et contribue aux risques d'infection et à la pollution environnementale. La mauvaise élimination et le recours croissant aux produits jetables comme les seringues et les cathéters exigent une attention urgente à la gestion durable des déchets dans les soins de santé maternelle.
- En décembre 2024, selon Centurial, si les dispositifs jetables de traitement de l'HPP améliorent la sécurité et réduisent les risques d'infection, leur utilisation généralisée soulève des préoccupations environnementales. Une élimination inappropriée contribue à l'accumulation de déchets médicaux et à la pollution. Il est essentiel de concilier le besoin d'outils stériles à usage unique et une gestion durable des déchets afin de minimiser les impacts environnementaux tout en garantissant des soins maternels efficaces.
Portée du marché européen des dispositifs de traitement des hémorragies post-partum
Le marché européen des dispositifs de traitement des hémorragies post-partum est classé en cinq segments notables qui sont basés sur le type, l'état, le type de patient, l'utilisateur final et le canal de distribution.
|
Segmentation |
Sous-segmentation |
|
Par type |
|
|
Par condition |
|
|
Par type de patient |
|
|
Par utilisateur final
|
|
|
Par canal de distribution |
|
Analyse régionale du marché européen des dispositifs de traitement des hémorragies post-partum
« L'Allemagne est le pays dominant sur le marché des dispositifs de traitement des hémorragies post-partum »
- L'Allemagne dispose d'un système de santé bien établi, offrant des soins maternels de haute qualité. Les politiques proactives du gouvernement et les investissements substantiels dans la santé maternelle contribuent à l'adoption généralisée de dispositifs avancés de traitement de l'HPP dans tout le pays.
- Le pays compte une population importante exposée à des pathologies associées à une HPP sévère, telles que les grossesses multiples, les fibromes utérins, l'anémie et le syndrome HELLP. Ce risque élevé stimule la demande de solutions efficaces pour le traitement de l'HPP, positionnant l'Allemagne comme un marché leader en Europe.
- L'Allemagne abrite des acteurs majeurs du secteur des dispositifs de traitement de l'HPP, notamment Cook Medical et Utah Medical Products. Ces entreprises sont très présentes sur le marché allemand et proposent une gamme de produits innovants, comme les tamponnements utérins par ballonnet, très demandés pour leur facilité d'utilisation et leur efficacité.
L'Allemagne devrait enregistrer le TCAC le plus élevé du marché .
- L'Allemagne compte une population importante exposée aux pathologies associées à une HPP sévère, telles que les grossesses multiples, les fibromes utérins, l'anémie et le syndrome HELLP. Ce risque élevé stimule la demande de solutions thérapeutiques efficaces pour l'HPP, positionnant l'Allemagne comme un marché leader en Europe.
- L'Allemagne dispose d'un système de santé bien établi, offrant des soins maternels de haute qualité. Les politiques proactives du gouvernement et les investissements substantiels dans la santé maternelle contribuent à l'adoption généralisée de dispositifs avancés de traitement de l'HPP dans tout le pays.
Part de marché des dispositifs de traitement des hémorragies post-partum
Le paysage concurrentiel du marché fournit des détails par concurrent. Il comprend la présentation de l'entreprise, ses données financières, son chiffre d'affaires, son potentiel de marché, ses investissements en recherche et développement, ses nouvelles initiatives commerciales, sa présence en Europe, ses sites et installations de production, ses capacités de production, ses forces et faiblesses, le lancement de nouveaux produits, leur ampleur et leur portée, ainsi que la domination de ses applications. Les données ci-dessus ne concernent que les activités des entreprises par rapport à leur marché.
Les principaux leaders du marché opérant sur le marché sont :
- BD (États-Unis)
- Groupe de sociétés Organon (Pays-Bas)
- Laborie (États-Unis)
- Cooper Companies (États-Unis)
- Belmont Medical Technologies (États-Unis)
- Utah Medical Products, Inc. (États-Unis)
- Angiplast Private Limited (Inde)
- Krishco Medical Products Pvt. Ltd. (Inde)
- 3rd Stone Design (États-Unis)
- Advin Health Care (États-Unis)
- Coagulant Therapeutics Corporation (États-Unis)
- Groupe Sterimed (États-Unis)
- RevMedx (États-Unis)
- Maternova Inc (États-Unis)
- Sinapi Biomedical (États-Unis)
Derniers développements dans les dispositifs de traitement des hémorragies post-partum
- En avril 2025, Organon a acquis auprès de Biogen les droits américains de TOFIDENCE, un biosimilaire du tocilizumab d'ACTEMRA. Cette acquisition renforce le portefeuille de biosimilaires d'Organon en immunologie et élargit les options thérapeutiques pour l'arthrite et la COVID-19. TOFIDENCE, lancé en mai 2024, traite de multiples affections inflammatoires et soutient la croissance de l'activité biosimilaires d'Organon, avec un potentiel de marché important.
- En novembre 2023, CooperCompanies a acquis certains actifs de Cook Medical pour 300 millions de dollars américains, enrichissant ainsi son portefeuille de produits de santé féminine et de chirurgie sous CooperSurgical. L'accord comprend des produits tels que le ballon Bakri et les moniteurs Doppler. Cette acquisition, qui devrait accroître le chiffre d'affaires et les bénéfices en 2024, renforce la position de Cooper en Europe dans les soins de fertilité et de gynécologie.
SKU-
Accédez en ligne au rapport sur le premier cloud mondial de veille économique
- Tableau de bord d'analyse de données interactif
- Tableau de bord d'analyse d'entreprise pour les opportunités à fort potentiel de croissance
- Accès d'analyste de recherche pour la personnalisation et les requêtes
- Analyse de la concurrence avec tableau de bord interactif
- Dernières actualités, mises à jour et analyse des tendances
- Exploitez la puissance de l'analyse comparative pour un suivi complet de la concurrence
Table des matières
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF THE EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 TYPE LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 VENDOR SHARE ANALYSIS
2.11 MARKET END USER COVERAGE GRID
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTAL ANALYSIS
4.2 PORTERS FIVE FORCES ANALYSIS
4.3 INDUSTRY INSIGHTS
4.3.1 MICRO AND MACROECONOMIC FACTORS
4.3.2 PENETRATION AND GROWTH PROSPECT MAPPING
4.3.3 KEY PRICING STRATEGIES
4.4 COST ANALYSIS BREAKDOWN
4.5 TECHNOLOGY ROADMAP
4.6 VALUE CHAIN ANALYSIS
4.7 OPPORTUNITY MAP ANALYSIS
4.8 HEALTHCARE ECONOMY
4.9 REIMBURSEMENT FRAMEWORK
4.1 TARIFFS AND ITS IMPACT ON THE MARKET
4.10.1 DEFINITION AND IMPORTANCE OF TARIFFS IN THE HEALTHCARE SECTOR
4.10.2 EUROPE VS. REGIONAL TARIFF STRUCTURES
4.10.3 IMPACT OF TARIFFS ON HEALTHCARE COSTS AND ACCESSIBILITY
4.10.4 TARIFF REGULATIONS IN KEY MARKETS
4.10.4.1 MEDICARE/MEDICAID TARIFF POLICIES
4.10.4.2 CMS PRICING MODELS
4.10.4.3 OTHERS
4.10.5 TARIFFS ON MEDICAL DEVICES & EQUIPMENT
4.10.5.1 IMPORT/EXPORT DUTIES ON MEDICAL EQUIPMENT
4.10.5.2 IMPACT ON PRICING AND AVAILABILITY OF HIGH-END MEDICAL TECHNOLOGY
4.10.5.3 CASE STUDIES OF TARIFF CHANGES AFFECTING THE INDUSTRY
4.10.6 COST BURDEN ON HOSPITALS AND HEALTHCARE FACILITIES
4.10.7 TARIFF EXEMPTIONS AND INCENTIVES
4.10.8 DUTY-FREE IMPORTS FOR ESSENTIAL MEDICINES AND VACCINES
4.10.9 IMPACT OF TRADE WARS ON THE HEALTHCARE SUPPLY CHAIN
4.10.10 ROLE OF FREE TRADE AGREEMENTS (FTAS) IN REDUCING TARIFFS
5 REGULATORY FRAMEWORK
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 RISING INCIDENCE OF POSTPARTUM HAEMORRHAGE
6.1.2 ONGOING TECHNOLOGICAL ADVANCEMENTS FOR POSTPARTUM HEMORRHAGE TREATMENTS
6.1.3 RISING BIRTH RATES ASSOCIATED WITH AN INCREASE IN THE NUMBER OF POSTPARTUM HEMORRHAGE
6.1.4 REGULATORY SUPPORT AND APPROVALS ASSOCIATED WITH THE TREATMENT DEVICES
6.2 RESTRAINTS
6.2.1 SIDE EFFECTS ASSOCIATED WITH THE POSTPARTUM HEMORRHAGE TREATMENT
6.2.2 LIMITED RESEARCH AND DEVELOPMENT FOR PPH TREATMENT
6.3 OPPORTUNITIES
6.3.1 TRAINING AND EDUCATIONAL PROGRAMS FOR PROPER USE OF PPH TREATMENT DEVICES
6.3.2 SUPPORT FROM GOVERNMENTAL AND NON-GOVERNMENTAL ORGANIZATIONS IN PPH DEVICE ADOPTION
6.3.3 TELEMEDICINE INTEGRATION TO ENHANCE POSTPARTUM HEMORRHAGE DEVICE USE
6.4 CHALLENGES
6.4.1 ENVIRONMENTAL IMPACT AND DISPOSAL ISSUES OF SINGLE-USE PPH DEVICES
6.4.2 STERILITY CHALLENGES AND INFECTION RISKS IN PPH DEVICES
7 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE
7.1 OVERVIEW
7.2 UTERINE BALLOON TAMPONADE
7.2.1 BAKRI BALLOON
7.2.1.1 BAKRI POSTPARTUM BALLOON
7.2.1.2 BAKRI POSTPARTUM BALLOON WITH RAPID INSTILLATION COMPONENTS
7.2.2 FOLEY CATHETER
7.2.2.1 STANDARD FOLEY CATHETER
7.2.2.2 CONDOM-LOADED FOLEY CATHETER
7.3 UNIJECT PREFILLED INJECTION SYSTEM
7.3.1 OXYTOCIN-BASED INJECTION SYSTEM
7.3.2 CARBETOCIN-BASED INJECTION SYSTEM
7.4 NON-PNEUMATIC ANTI-SHOCK GARMENT
7.4.1 STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT
7.4.1.1 MEDIUM
7.4.1.2 LARGE
7.4.2 MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT
7.4.2.1 MEDIUM
7.4.2.2 LARGE
7.5 VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES
7.5.1 JADA SYSTEM
7.5.2 OTHERS
7.6 OTHERS
7.6.1 COMPRESSION DEVICES
7.6.1.1 B-LYNCH
7.6.1.2 HAYMAN
7.6.1.3 OTHER
7.6.2 UTERINE ARTERY LIGATION PRODUCTS
7.6.3 OTHERS
8 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE
8.1 OVERVIEW
8.2 PRIMARY PPH
8.3 SECONDARY PPH
9 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION
9.1 OVERVIEW
9.2 MAJOR POSTPARTUM HEMORRHAGE (MORE THAN 1000 ML)
9.3 MINOR POSTPARTUM HEMORRHAGE (500-1000 ML)
9.4 MASSIVE POSTPARTUM HEMORRHAGE (2000 ML OR MORE)
9.5 SECONDARY POSTPARTUM HEMORRHAGE
10 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL
10.1 OVERVIEW
10.2 DIRECT TENDER
10.3 RETAIL SALES
10.3.1 OFFLINE SALES
10.3.2 ONLINE SALES
10.4 OTHERS
11 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER
11.1 OVERVIEW
11.2 HOSPITALS
11.2.1 PUBLIC HOSPITALS
11.2.1.1 TIER 2
11.2.1.2 TIER 3
11.2.1.3 TIER 1
11.2.2 PRIVATE HOSPITALS
11.2.2.1 TIER 2
11.2.2.2 TIER 3
11.2.2.3 TIER 1
11.3 MATERNITY CENTERS
11.4 SPECIALTY CLINICS
11.5 HOME CARE SETTINGS
11.6 OTHERS
12 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION
12.1 EUROPE
12.1.1 GERMANY
12.1.2 FRANCE
12.1.3 U.K.
12.1.4 ITALY
12.1.5 SPAIN
12.1.6 RUSSIA
12.1.7 TURKEY
12.1.8 NETHERLAND
12.1.9 POLAND
12.1.10 BELGIUM
12.1.11 SWITZERLAND
12.1.12 SWEDEN
12.1.13 DENMARK
12.1.14 NORWAY
12.1.15 FINLAND
12.1.16 REST OF EUROPE
13 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: COMPANY LANDSCAPE
13.1 COMPANY SHARE ANALYSIS: GLOBAL
14 SWOT ANALYSIS
15 COMPANY PROFILES
15.1 BD
15.1.1 COMPANY SNAPSHOT
15.1.2 REVENUE ANALYSIS
15.1.3 COMPANY SHARE ANALYSIS
15.1.4 SOLUTION PORTFOLIO
15.1.5 RECENT NEWS
15.2 ORGANON GROUP OF COMPANIES
15.2.1 COMPANY SNAPSHOT
15.2.2 REVENUE ANALYSIS
15.2.3 COMPANY SHARE ANALYSIS
15.2.4 PRODUCT PORTFOLIO
15.2.5 RECENT DEVELOPMENTS/NEWS
15.3 LABORIE
15.3.1 COMPANY SNAPSHOT
15.3.2 COMPANY SHARE ANALYSIS
15.3.3 PRODUCT PORTFOLIO
15.3.4 RECENT DEVELOPMENT
15.4 COOPERCOMPANIES
15.4.1 COMPANY SNAPSHOT
15.4.2 REVENUE ANALYSIS
15.4.3 COMPANY SHARE ANALYSIS
15.4.4 PRODUCT PORTFOLIO
15.4.5 RECENT DEVELOPMENT
15.5 BELMONT MEDICAL TECHNOLOGIES
15.5.1 COMPANY SNAPSHOT
15.5.2 COMPANY SHARE ANALYSIS
15.5.3 PRODUCT PORTFOLIO
15.5.4 RECENT DEVELOPMENT
15.6 ADVIN HEALTH CARE
15.6.1 COMPANY SNAPSHOT
15.6.2 PRODUCT PORTFOLIO
15.6.3 RECENT DEVELOPMENTS
15.7 ANGIPLAST PRIVATE LIMITED
15.7.1 COMPANY SNAPSHOT
15.7.2 PRODUCT PORTFOLIO
15.7.3 RECENT DEVELOPMENT
15.8 COAGULANT THERAPEUTICS
15.8.1 COMPANY SNAPSHOT
15.8.2 PIPELINE PORTFOLIO
15.8.3 RECENT DEVELOPMENT
15.9 KRISHCO MEDICAL PRODUCTS PVT. LTD
15.9.1 COMPANY SNAPSHOT
15.9.2 PRODUCT PORTFOLIO
15.9.3 RECENT DEVELOPMENT
15.1 MATERNOVA INC.
15.10.1 COMPANY SNAPSHOT
15.10.2 PRODUCT PORTFOLIO
15.10.3 RECENT DEVELOPMENT
15.11 REVMEDX
15.11.1 COMPANY SNAPSHOT
15.11.2 PRODUCT PORTFOLIO
15.11.3 RECENT DEVELOPMENT
15.12 3RD STONE DESIGN
15.12.1 COMPANY SNAPSHOT
15.12.2 PRODUCT PORTFOLIO
15.12.3 RECENT DEVELOPMENTS
15.13 STERIMED GROUP
15.13.1 COMPANY SNAPSHOT
15.13.2 PRODUCT PORTFOLIO
15.13.3 RECENT DEVELOPMENTS
15.14 UTAH MEDICAL PRODUCTS, INC.
15.14.1 COMPANY SNAPSHOT
15.14.2 REVENUE ANALYSIS
15.14.3 PRODUCT PORTFOLIO
15.14.4 RECENT DEVELOPMENTS
15.15 SINAPI BIOMEDICAL
15.15.1 COMPANY SNAPSHOT
15.15.2 PRODUCT PORTFOLIO
15.15.3 RECENT DEVELOPMENTS
16 QUESTIONNAIRE
Liste des tableaux
TABLE 1 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 2 EUROPE UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 3 EUROPE UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 4 EUROPE BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 5 EUROPE FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 6 EUROPE UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 7 EUROPE UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 8 EUROPE NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 9 EUROPE NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 10 EUROPE STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 11 EUROPE MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 12 EUROPE VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 13 EUROPE VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 14 EUROPE OTHERS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 15 EUROPE OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 16 EUROPE COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 17 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 18 EUROPE PRIMARY PPH IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 19 EUROPE SECONDARY PPH IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 20 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 21 EUROPE MAJOR POSTPARTUM HEMORRHAGE (MORE THAN 1000 ML) IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 22 EUROPE MINOR POSTPARTUM HEMORRHAGE (500-1000 ML) IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 23 EUROPE MASSIVE POSTPARTUM HEMORRHAGE (2000 ML OR MORE) IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 24 EUROPE SECONDARY POSTPARTUM HEMORRHAGE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 25 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 26 EUROPE DIRECT TENDER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 27 EUROPE RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 28 EUROPE RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 29 EUROPE OTHERS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 30 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 31 EUROPE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 32 EUROPE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 33 EUROPE PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 34 EUROPE PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 35 EUROPE MATERNITY CENTERS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 36 EUROPE SPECIALTY CLINICS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 37 EUROPE HOME CARE SETTINGS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 38 EUROPE OTHERS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 39 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY COUNTRY, 2018-2032 (USD THOUSAND)
TABLE 40 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 41 EUROPE UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 42 EUROPE BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 43 EUROPE FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 44 EUROPE UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 45 EUROPE NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 46 EUROPE STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 47 EUROPE MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 48 EUROPE VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 49 EUROPE OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 50 EUROPE COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 51 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 52 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 53 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 54 EUROPE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 55 EUROPE PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 56 EUROPE PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 57 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 58 EUROPE RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 59 GERMANY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 60 GERMANY UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 61 GERMANY BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 62 GERMANY FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 63 GERMANY UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 64 GERMANY NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 65 GERMANY STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 66 GERMANY MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 67 GERMANY VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 68 GERMANY OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 69 GERMANY COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 70 GERMANY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 71 GERMANY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 72 GERMANY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 73 GERMANY HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 74 GERMANY PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 75 GERMANY PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 76 GERMANY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 77 GERMANY RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 78 FRANCE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 79 FRANCE UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 80 FRANCE BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 81 FRANCE FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 82 FRANCE UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 83 FRANCE NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 84 FRANCE STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 85 FRANCE MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 86 FRANCE VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 87 FRANCE OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 88 FRANCE COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 89 FRANCE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 90 FRANCE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 91 FRANCE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 92 FRANCE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 93 FRANCE PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 94 FRANCE PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 95 FRANCE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 96 FRANCE RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 97 U.K. POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 98 U.K. UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 99 U.K. BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 100 U.K. FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 101 U.K. UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 102 U.K. NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 103 U.K. STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 104 U.K. MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 105 U.K. VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 106 U.K. OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 107 U.K. COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 108 U.K. POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 109 U.K. POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 110 U.K. POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 111 U.K. HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 112 U.K. PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 113 U.K. PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 114 U.K. POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 115 U.K. RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 116 ITALY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 117 ITALY UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 118 ITALY BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 119 ITALY FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 120 ITALY UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 121 ITALY NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 122 ITALY STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 123 ITALY MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 124 ITALY VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 125 ITALY OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 126 ITALY COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 127 ITALY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 128 ITALY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 129 ITALY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 130 ITALY HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 131 ITALY PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 132 ITALY PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 133 ITALY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 134 ITALY RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 135 SPAIN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 136 SPAIN UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 137 SPAIN BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 138 SPAIN FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 139 SPAIN UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 140 SPAIN NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 141 SPAIN STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 142 SPAIN MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 143 SPAIN VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 144 SPAIN OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 145 SPAIN COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 146 SPAIN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 147 SPAIN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 148 SPAIN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 149 SPAIN HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 150 SPAIN PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 151 SPAIN PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 152 SPAIN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 153 SPAIN RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 154 RUSSIA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 155 RUSSIA UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 156 RUSSIA BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 157 RUSSIA FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 158 RUSSIA UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 159 RUSSIA NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 160 RUSSIA STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 161 RUSSIA MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 162 RUSSIA VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 163 RUSSIA OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 164 RUSSIA COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 165 RUSSIA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 166 RUSSIA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 167 RUSSIA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 168 RUSSIA HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 169 RUSSIA PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 170 RUSSIA PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 171 RUSSIA POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 172 RUSSIA RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 173 TURKEY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 174 TURKEY UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 175 TURKEY BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 176 TURKEY FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 177 TURKEY UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 178 TURKEY NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 179 TURKEY STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 180 TURKEY MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 181 TURKEY VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 182 TURKEY OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 183 TURKEY COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 184 TURKEY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 185 TURKEY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 186 TURKEY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 187 TURKEY HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 188 TURKEY PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 189 TURKEY PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 190 TURKEY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 191 TURKEY RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 192 NETHERLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 193 NETHERLAND UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 194 NETHERLAND BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 195 NETHERLAND FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 196 NETHERLAND UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 197 NETHERLAND NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 198 NETHERLAND STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 199 NETHERLAND MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 200 NETHERLAND VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 201 NETHERLAND OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 202 NETHERLAND COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 203 NETHERLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 204 NETHERLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 205 NETHERLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 206 NETHERLAND HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 207 NETHERLAND PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 208 NETHERLAND PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 209 NETHERLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 210 NETHERLAND RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 211 POLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 212 POLAND UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 213 POLAND BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 214 POLAND FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 215 POLAND UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 216 POLAND NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 217 POLAND STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 218 POLAND MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 219 POLAND VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 220 POLAND OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 221 POLAND COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 222 POLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 223 POLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 224 POLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 225 POLAND HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 226 POLAND PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 227 POLAND PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 228 POLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 229 POLAND RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 230 BELGIUM POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 231 BELGIUM UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 232 BELGIUM BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 233 BELGIUM FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 234 BELGIUM UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 235 BELGIUM NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 236 BELGIUM STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 237 BELGIUM MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 238 BELGIUM VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 239 BELGIUM OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 240 BELGIUM COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 241 BELGIUM POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 242 BELGIUM POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 243 BELGIUM POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 244 BELGIUM HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 245 BELGIUM PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 246 BELGIUM PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 247 BELGIUM POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 248 BELGIUM RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 249 SWITZERLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 250 SWITZERLAND UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 251 SWITZERLAND BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 252 SWITZERLAND FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 253 SWITZERLAND UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 254 SWITZERLAND NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 255 SWITZERLAND STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 256 SWITZERLAND MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 257 SWITZERLAND VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 258 SWITZERLAND OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 259 SWITZERLAND COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 260 SWITZERLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 261 SWITZERLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 262 SWITZERLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 263 SWITZERLAND HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 264 SWITZERLAND PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 265 SWITZERLAND PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 266 SWITZERLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 267 SWITZERLAND RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 268 SWEDEN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 269 SWEDEN UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 270 SWEDEN BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 271 SWEDEN FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 272 SWEDEN UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 273 SWEDEN NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 274 SWEDEN STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 275 SWEDEN MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 276 SWEDEN VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 277 SWEDEN OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 278 SWEDEN COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 279 SWEDEN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 280 SWEDEN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 281 SWEDEN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 282 SWEDEN HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 283 SWEDEN PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 284 SWEDEN PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 285 SWEDEN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 286 SWEDEN RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 287 DENMARK POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 288 DENMARK UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 289 DENMARK BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 290 DENMARK FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 291 DENMARK UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 292 DENMARK NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 293 DENMARK STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 294 DENMARK MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 295 DENMARK VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 296 DENMARK OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 297 DENMARK COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 298 DENMARK POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 299 DENMARK POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 300 DENMARK POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 301 DENMARK HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 302 DENMARK PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 303 DENMARK PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 304 DENMARK POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 305 DENMARK RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 306 NORWAY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 307 NORWAY UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 308 NORWAY BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 309 NORWAY FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 310 NORWAY UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 311 NORWAY NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 312 NORWAY STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 313 NORWAY MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 314 NORWAY VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 315 NORWAY OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 316 NORWAY COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 317 NORWAY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 318 NORWAY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 319 NORWAY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 320 NORWAY HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 321 NORWAY PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 322 NORWAY PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 323 NORWAY POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 324 NORWAY RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 325 FINLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 326 FINLAND UTERINE BALLOON TAMPONADE IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 327 FINLAND BAKRI BALLOON IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 328 FINLAND FOLEY CATHETER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 329 FINLAND UNIJECT PREFILLED INJECTION SYSTEM IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 330 FINLAND NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 331 FINLAND STANDARD NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 332 FINLAND MODIFIED NON-PNEUMATIC ANTI-SHOCK GARMENT IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY SIZE, 2018-2032 (USD THOUSAND)
TABLE 333 FINLAND VACUUM-INDUCED HEMORRHAGE CONTROL DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 334 FINLAND OTHER IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 335 FINLAND COMPRESSION DEVICES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 336 FINLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY CONDITION, 2018-2032 (USD THOUSAND)
TABLE 337 FINLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY PATIENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 338 FINLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 339 FINLAND HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 340 FINLAND PUBLIC HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 341 FINLAND PRIVATE HOSPITALS IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 342 FINLAND POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 343 FINLAND RETAIL SALES IN POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 344 REST OF EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
Liste des figures
FIGURE 1 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: SEGMENTATION
FIGURE 2 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: DATA TRIANGULATION
FIGURE 3 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: DROC ANALYSIS
FIGURE 4 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: EUROPE VS REGIONAL MARKET ANALYSIS
FIGURE 5 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: DBMR MARKET POSITION GRID
FIGURE 8 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: VENDOR SHARE ANALYSIS
FIGURE 9 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: MARKET END USER COVERAGE GRID
FIGURE 10 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: SEGMENTATION
FIGURE 11 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: EXECUTIVE SUMMARY
FIGURE 12 STRATEGIC DECISIONS
FIGURE 13 RISING INCIDENCE OF POSTPARTUM HAEMORRHAGE IS EXPECTED TO DRIVE THE EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET IN THE FORECAST PERIOD OF 2025 TO 2032
FIGURE 14 UTERINE BALLOON TAMPONADE SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET IN THE FORECAST PERIOD OF 2025 & 2032
FIGURE 15 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET, BY TYPE (2024)
FIGURE 16 DRIVERS, RESTRAINTS, OPPORTUNITIES AND CHALLENGES OF EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET
FIGURE 17 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY TYPE, 2024
FIGURE 18 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY TYPE, 2025-2032 (USD THOUSAND)
FIGURE 19 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY TYPE, CAGR (2025-2032)
FIGURE 20 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY TYPE, LIFELINE CURVE
FIGURE 21 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY PATIENT TYPE, 2024
FIGURE 22 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY PATIENT TYPE, 2025-2032 (USD THOUSAND)
FIGURE 23 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY PATIENT TYPE, CAGR (2025-2032)
FIGURE 24 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY PATIENT TYPE, LIFELINE CURVE
FIGURE 25 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY CONDITION, 2024
FIGURE 26 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY CONDITION, 2025-2032 (USD THOUSAND)
FIGURE 27 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY CONDITION, CAGR (2025-2032)
FIGURE 28 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY CONDITION, LIFELINE CURVE
FIGURE 29 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY DISTRIBUTION CHANNEL, 2024
FIGURE 30 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY DISTRIBUTION CHANNEL, 2025-2032 (USD THOUSAND)
FIGURE 31 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY DISTRIBUTION CHANNEL, CAGR (2025-2032)
FIGURE 32 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 33 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY END USER, 2024
FIGURE 34 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY END USER, 2025-2032 (USD THOUSAND)
FIGURE 35 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY END USER, CAGR (2025-2032)
FIGURE 36 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: BY END USER, LIFELINE CURVE
FIGURE 37 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: SNAPSHOT (2024)
FIGURE 38 EUROPE POSTPARTUM HEMORRHAGE TREATMENT DEVICES MARKET: COMPANY SHARE 2024 (%)

Méthodologie de recherche
La collecte de données et l'analyse de l'année de base sont effectuées à l'aide de modules de collecte de données avec des échantillons de grande taille. L'étape consiste à obtenir des informations sur le marché ou des données connexes via diverses sources et stratégies. Elle comprend l'examen et la planification à l'avance de toutes les données acquises dans le passé. Elle englobe également l'examen des incohérences d'informations observées dans différentes sources d'informations. Les données de marché sont analysées et estimées à l'aide de modèles statistiques et cohérents de marché. De plus, l'analyse des parts de marché et l'analyse des tendances clés sont les principaux facteurs de succès du rapport de marché. Pour en savoir plus, veuillez demander un appel d'analyste ou déposer votre demande.
La méthodologie de recherche clé utilisée par l'équipe de recherche DBMR est la triangulation des données qui implique l'exploration de données, l'analyse de l'impact des variables de données sur le marché et la validation primaire (expert du secteur). Les modèles de données incluent la grille de positionnement des fournisseurs, l'analyse de la chronologie du marché, l'aperçu et le guide du marché, la grille de positionnement des entreprises, l'analyse des brevets, l'analyse des prix, l'analyse des parts de marché des entreprises, les normes de mesure, l'analyse globale par rapport à l'analyse régionale et des parts des fournisseurs. Pour en savoir plus sur la méthodologie de recherche, envoyez une demande pour parler à nos experts du secteur.
Personnalisation disponible
Data Bridge Market Research est un leader de la recherche formative avancée. Nous sommes fiers de fournir à nos clients existants et nouveaux des données et des analyses qui correspondent à leurs objectifs. Le rapport peut être personnalisé pour inclure une analyse des tendances des prix des marques cibles, une compréhension du marché pour d'autres pays (demandez la liste des pays), des données sur les résultats des essais cliniques, une revue de la littérature, une analyse du marché des produits remis à neuf et de la base de produits. L'analyse du marché des concurrents cibles peut être analysée à partir d'une analyse basée sur la technologie jusqu'à des stratégies de portefeuille de marché. Nous pouvons ajouter autant de concurrents que vous le souhaitez, dans le format et le style de données que vous recherchez. Notre équipe d'analystes peut également vous fournir des données sous forme de fichiers Excel bruts, de tableaux croisés dynamiques (Fact book) ou peut vous aider à créer des présentations à partir des ensembles de données disponibles dans le rapport.