Europe Traumatic Brain Injury Treatment Market
Taille du marché en milliards USD
TCAC :
% 
 USD
1.01 Billion
USD
1.60 Billion
2024
2032
USD
1.01 Billion
USD
1.60 Billion
2024
2032
| 2025 –2032 | |
| USD 1.01 Billion | |
| USD 1.60 Billion | |
|
|
|
Segmentation du marché européen du traitement des traumatismes crâniens, par traitement (chirurgie, soins d'urgence immédiats et médicaments), voie d'administration (parentérale, orale et autres), âge du patient (enfants, adolescents et personnes âgées), sexe (homme et femme), cause de blessure (chutes, circulation automobile, sports et autres), utilisateur final (hôpitaux, cliniques neurologiques, pharmacies indépendantes et autres) - Tendances et prévisions du secteur jusqu'en 2032
Taille du marché du traitement des lésions cérébrales traumatiques
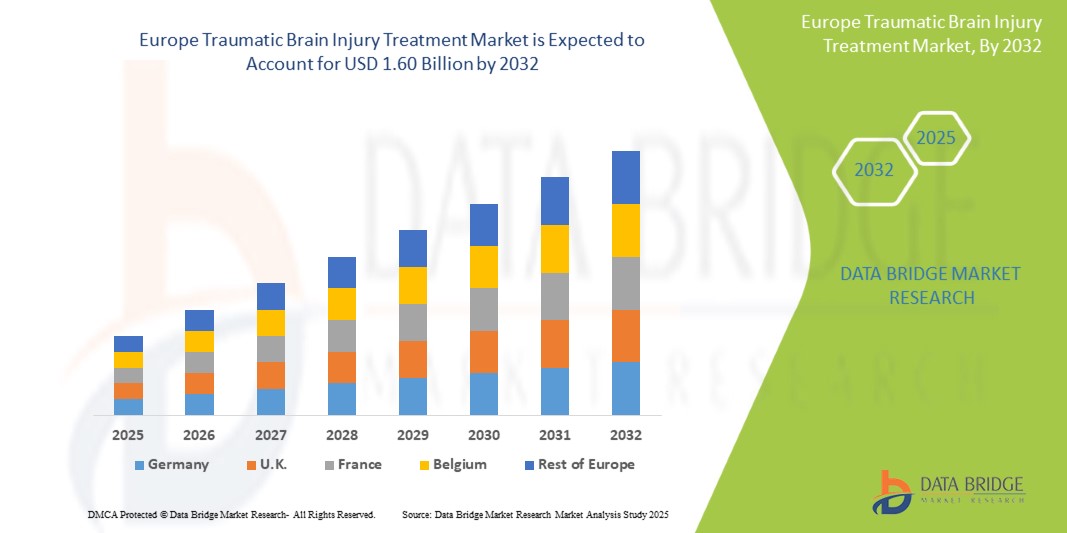
- Le marché européen du traitement des traumatismes crâniens était évalué à 1,01 milliard USD en 2024 et devrait atteindre 1,60 milliard USD d'ici 2032.
- Au cours de la période de prévision de 2025 à 2032, le marché devrait croître à un TCAC de 6,0 %, principalement en raison de l'incidence croissante des traumatismes crâniens (TCC).
- Cette croissance est due à des facteurs tels que l’incidence croissante des lésions cérébrales traumatiques (LCT), l’adoption croissante de procédures mini-invasives dans le traitement des LCT et la demande croissante de traitement des lésions cérébrales traumatiques.
Analyse du marché du traitement des traumatismes crâniens
- Le marché du traitement des lésions cérébrales traumatiques (LCT) devrait connaître une croissance significative en raison de la sensibilisation croissante aux LCT, des progrès des technologies de diagnostic et de l'incidence croissante des accidents et des blessures liées au sport, ce qui stimule la demande d'options de traitement efficaces et de thérapies de réadaptation.
- Le marché connaît une augmentation des traitements innovants, notamment des agents neuroprotecteurs, des thérapies à base de cellules souches et des technologies de réadaptation avancées, qui améliorent les résultats de la récupération et élargissent le paysage thérapeutique pour les patients atteints de lésions cérébrales traumatiques.
- L'Allemagne devrait être le leader du marché européen du traitement des lésions cérébrales traumatiques (LCT), grâce à son infrastructure de soins de santé avancée et à ses investissements importants dans la recherche et le développement médicaux.
Portée du rapport et segmentation du marché du traitement des lésions cérébrales traumatiques
|
Attributs |
Aperçu du marché du traitement des traumatismes crâniens |
|
Segments couverts |
|
|
Pays couverts |
Europe
|
|
Principaux acteurs du marché |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include import export analysis, production capacity overview, production consumption analysis, price trend analysis, climate change scenario, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Traumatic Brain Injury Treatment Market Trends
“Growing Adoption of Telemedicine in TBI Treatment”
- Telemedicine allows patients to receive remote consultations and rehabilitation services, improving access to specialized care for individuals in rural or underserved areas, thus facilitating timely intervention for TBI patients
- Utilizing telehealth reduces the costs associated with in-person visits, including travel expenses and lost productivity, making it a financially viable option for both patients and healthcare providers
- In March 2021, NCBI stated that Telehealth visits for patients with acquired brain injuries and their caregivers can ease the burden of transportation, improve compliance, and increase overall satisfaction. Management strategies are largely unaffected in the telehealth setting, and telerehab options have been found to be equal or superior to in-person therapy to treat many associated deficits
- Telemedicine platforms enable continuous monitoring and follow-up care, allowing healthcare professionals to track patient progress remotely, provide real-time feedback, and adjust treatment plans as necessary, ultimately enhancing patient outcomes in TBI management
Traumatic Brain Injury Treatment Market Dynamics
Driver
“Increasing Incidence of Traumatic Brain Injury (TBI)”
- As the number of cases continues to rise due to various contributing factors such as road accidents, sports-related injuries, and falls, particularly among the elderly population. Road traffic accidents remain one of the leading causes of TBI worldwide, with the growing number of vehicles on the road, reckless driving behaviours
- Sports-related injuries, particularly in contact sports like football, boxing, and rugby, have further fueled the surge in TBI cases, with increasing awareness of concussion-related complications prompting the need for advanced treatment solutions
For instance,
- In March 2025, as per the article published by ScienceDirect, there were 20.84 million incident cases and 37.93 million prevalent cases of Traumatic Brain Injury (TBI) Europely, leading to 5.48 million, Years Lived With Disability (YLDs). The rising burden of TBI increases the demand for advanced treatments, driving investments in diagnostics, neurosurgery, and rehabilitation, ultimately fueling the growth of the Europe TBI treatment market
- In October 2024, according to the data published by Centers for Disease Control and Prevention, In 2021, there were 69,473 TBI-related deaths, and in 2020, approximately 214,110 hospitalizations occurred. This equates to over 586 hospitalizations and 190 deaths per day, with individuals aged 75+ and males being the most affected. The rising burden of TBI necessitates advanced treatment solutions, driving growth in the Europe TBI treatment market
- Factors such as road accidents, sports injuries, and falls—especially among the elderly—contribute to increase in TBI. Growing awareness and demand for advanced treatments, including neurosurgery, drug therapies, and rehabilitation, are fuelling market expansion and technological advancements in TBI management
Opportunity
“Rising Personalized and Targeted Therapies in Traumatic Brain Injury (TBI)”
- TBI is a highly variable condition influenced by severity, location, and patient-specific factors, making traditional treatments less effective. Advances in biomarker discovery, neuroimaging, and computational modeling help identify distinct injury patterns, enabling more targeted therapies. Pharmacogenomics enhances drug selection and dosing, minimizing side effects while maximizing effectiveness. Personalized rehabilitation strategies, tailored to cognitive and motor impairments, further optimize recovery by aligning treatments with individual healing trajectories
For instance,
- In February 2022, as per NCBI, researchers have discovered genetic risk factors like APOE4 and BDNF Val66Met polymorphisms that impact TBI recovery. By focusing on these variations, personalized treatments can lower harmful biomarkers, enhance neuroprotection, and improve rehabilitation. This approach tailors’ therapies to individual needs, ultimately leading to better long-term functional outcomes for TBI patients
- In February 2024, article by MDPI TBI presents a significant opportunity to enhance patient outcomes. Advancements in biomarker discovery, pharmacogenomics, and neuroimaging enable precision treatments tailored to individual injury profiles. Emerging therapies, including neurostimulation and stem cell treatments, further expand possibilities for effective, patient-specific interventions in TBI management
- Personalized and targeted therapies present a transformative approach to managing Traumatic Brain Injury (TBI) by tailoring interventions to individual genetic and molecular profiles. These strategies focus on specific biomarkers and cellular processes to reduce secondary damage and improve recovery. By optimizing treatment, personalized therapies enhance outcomes and promote long-term functional recovery for TBI patients.
Restraint/Challenge
“Difficulties in Overcoming the Blood-Brain Barrier for TBI Treatment”
- A significant challenge in Traumatic Brain Injury (TBI) treatment is the disruption of the Blood-Brain Barrier (BBB). After a TBI, the Blood-Brain Barrier (BBB )often becomes compromised, allowing harmful substances to enter the brain, which can worsen injury and hinder recovery. This creates difficulty in delivering therapeutic agents effectively, limiting the success of many treatments designed to aid recovery and protect brain tissue.
- Furthermore, restoring the integrity of the Blood-Brain Barrier (BBB) without causing additional harm remains a major challenge. Developing targeted delivery systems that can bypass the damaged barrier without introducing further risks is crucial for improving TBI treatment outcomes.
For instance,
- In January 2022, Springer Nature Publishing Inc reported that Blood-Brain Barrier (BBB) it restricts the delivery of therapeutic agents to the brain. Even when the BBB is compromised after injury, many drugs, particularly large molecules, still struggle to penetrate it, limiting the effectiveness of treatments and complicating targeted therapies.
- In June 2024, nature reviews neurology reported that BBB dysfunction can persist from days to years after TBI, contributing to long-term neurological complications. This dysfunction is linked to oedema, neuroinflammation, and alterations in neuronal networks, complicating treatment strategies and leading to cognitive impairments, depression, and post-traumatic epilepsy, thus challenging effective recovery and therapeutic approaches
- The Blood-Brain Barrier (BBB) disruption presents a significant challenge in treating Traumatic Brain Injury (TBI), as it restricts the effective delivery of treatments and aggravates brain damage. Persistent BBB dysfunction can lead to long-term complications, including inflammation, brain swelling, and cognitive disorders. While approaches like ROS-scavenging therapy show promise in improving brain function, the fluctuating nature of BBB damage complicates therapeutic strategies. To improve TBI outcomes, there is a critical need for innovative drug delivery systems and better methods for monitoring BBB integrity, enabling more effective treatments and reducing long-term neurological impairments
Traumatic Brain Injury Treatment Market Scope
The market is segmented on the basis of treatment, patient age, gender, cause of injury, and end user.
|
Segmentation |
Sub-Segmentation |
|
By Treatment |
|
|
By Patient Age |
|
|
Par sexe |
|
|
Par cause de blessure |
|
|
Par utilisateur final |
|
Analyse régionale du marché du traitement des traumatismes crâniens
« L'Allemagne est le pays dominant sur le marché du traitement des traumatismes crâniens »
- L'Allemagne devrait devenir le leader du marché européen du traitement des lésions cérébrales traumatiques (LCT), grâce à son infrastructure de soins de santé avancée et à ses investissements importants dans la recherche et le développement médicaux.
- Le pays dispose d’un réseau bien établi d’hôpitaux neurologiques spécialisés, de centres de réadaptation et d’instituts de recherche qui se concentrent sur les lésions cérébrales et les affections connexes.
- De plus, les politiques de santé de l'Allemagne soutiennent des soins de haute qualité aux patients, garantissant un diagnostic précoce et des options de traitement efficaces, ce qui renforce encore sa position de leader du marché dans le traitement des lésions cérébrales traumatiques.
« L'Allemagne devrait enregistrer le taux de croissance le plus élevé »
- L'Allemagne devrait connaître le taux de croissance annuel composé (TCAC) le plus élevé de ce marché, grâce à une sensibilisation croissante aux stratégies de gestion des lésions cérébrales traumatiques et à l'adoption de modalités de traitement innovantes.
- Les campagnes de sensibilisation du public et les initiatives gouvernementales croissantes ont permis une meilleure reconnaissance des lésions cérébrales traumatiques, ce qui a permis des interventions plus précoces et de meilleurs résultats pour les patients.
- En outre, la forte présence de l’Allemagne dans les domaines de la biotechnologie et des produits pharmaceutiques a accéléré le développement de traitements de pointe, tels que les thérapies régénératives et les médicaments neuroprotecteurs, contribuant ainsi à la croissance rapide du marché.
Part de marché du traitement des lésions cérébrales traumatiques
Le paysage concurrentiel du marché fournit des détails par concurrent. Il comprend la présentation de l'entreprise, ses données financières, son chiffre d'affaires, son potentiel de marché, ses investissements en recherche et développement, ses nouvelles initiatives commerciales, sa présence en Europe, ses sites et installations de production, ses capacités de production, ses forces et faiblesses, le lancement de nouveaux produits, leur ampleur et leur portée, ainsi que la domination de ses applications. Les données ci-dessus ne concernent que les activités des entreprises par rapport à leur marché.
Les principaux leaders du marché opérant sur le marché sont :
- Pfizer Inc. (États-Unis)
- Teva Pharmaceuticals US, Inc. (États-Unis)
- Fresenius SE & Co. KGaA (Fresenius Kabi AG) (Allemagne)
- Viatris Inc. (États-Unis)
- Amneal Pharmaceuticals LLC. (États-Unis)
- Dr. Reddy's Laboratories Ltd. (Inde)
- Sun Pharmaceutical Industries, Inc. (Inde)
- Lupin (Inde)
- Hikma (Jordanie)
- Aurobindo Pharma US (Inde)
- ICU Medical (États-Unis)
- B. Braun Medical Inc. (Allemagne)
- Alembic Pharmaceuticals Limited (Inde)
- Merz Therapeutics (Allemagne)
- Advacare (Afrique du Sud)
- Maxzimaa (Inde)
- Jedux Parenteral Private Limited (Inde)
- Sagent Pharmaceuticals, Inc. (États-Unis)
- Swiss Pharma Nigeria Limited (Nigéria)
Dernières évolutions du marché du traitement des traumatismes crâniens
- En février 2024, Viatris et Idorsia ont conclu un important partenariat de recherche et développement en Amérique du Nord afin de développer des thérapies innovantes dans de multiples domaines thérapeutiques. Ce partenariat s'appuie sur l'expertise d'Idorsia en découverte de médicaments et sur la présence de Viatris en Amérique du Nord, accélérant le développement de traitements révolutionnaires et élargissant les portefeuilles de produits des deux entreprises, renforçant ainsi leur engagement à répondre aux besoins médicaux non satisfaits dans le monde entier.
- En février 2021, Fresenius Kabi a agrandi ses installations en Autriche, renforçant ainsi ses capacités de production et son innovation dans les domaines pharmaceutique et des technologies médicales. Cette expansion améliore l'efficacité de la production, assure un approvisionnement régulier en produits de soins intensifs et soutient les avancées de la recherche. En augmentant ses capacités et son excellence opérationnelle, l'entreprise renforce sa présence sur le marché et répond à la demande croissante de solutions de santé en Amérique du Nord.
SKU-
Accédez en ligne au rapport sur le premier cloud mondial de veille économique
- Tableau de bord d'analyse de données interactif
- Tableau de bord d'analyse d'entreprise pour les opportunités à fort potentiel de croissance
- Accès d'analyste de recherche pour la personnalisation et les requêtes
- Analyse de la concurrence avec tableau de bord interactif
- Dernières actualités, mises à jour et analyse des tendances
- Exploitez la puissance de l'analyse comparative pour un suivi complet de la concurrence
Table des matières
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF THE EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.8 DBMR MARKET POSITION GRID
2.9 MARKET END-USER COVERAGE GRID
2.1 VENDOR SHARE ANALYSIS
2.11 SECONDARY SOURCES
2.12 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PORTER’S FIVE FORCES
4.2 PESTEL ANALYSIS
4.3 TECHNOLOGY ROADMAP
4.4 VALUE CHAIN ANALYSIS
4.5 OPPUTUNITY MAP ANALYSIS
4.6 REIMBURSEMENT FRAMEWORK
4.7 COST ANALYSIS BREAKDOWN
4.8 PENETRATION AND GROWTH PROSPECT MAPPING: EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET
4.9 KEY PRICING STRATEGIES: EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET
4.1 MICRO AND MACRO-ECONOMIC FACTORS: EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET
5 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET, REGULATORY FRAMEWORK
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 INCREASING INCIDENCE OF TRAUMATIC BRAIN INJURY (TBI)
6.1.2 GROWING ADOPTION OF MINIMALLY INVASIVE PROCEDURES IN TBI TREATMENT
6.1.3 TECHNOLOGICAL ADVANCEMENT FOR DIAGNOSIS OF TRAUMATIC BRAIN INJURIES (TBI)
6.1.4 ADVANCEMENTS IN NEUROPROTECTION AND PHARMACOTHERAPY FOR TBI TREATMENT
6.2 RESTRAINTS
6.2.1 SHORTAGE OF TRAINED NEUROLOGISTS AND NEUROSURGEONS
6.2.2 HIGH COST OF TRAUMATIC BRAIN INJURY (TBI) TREATMENT
6.3 OPPORTUNITIES
6.3.1 RISING PERSONALIZED & TARGETED THERAPIES IN TRAUMATIC BRAIN INJURY (TBI)
6.3.2 GROWING BRAIN STIMULATION TECHNIQUES IN TRAUMATIC BRAIN INJURY (TBI) TREATMENT
6.3.3 RISING ARTIFICIAL INTELLIGENCE (AI) APPLICATIONS IN DIAGNOSING TRAUMATIC BRAIN INJURY (TBI)
6.4 CHALLENGES
6.4.1 DIFFICULTIES IN OVERCOMING THE BLOOD-BRAIN BARRIER FOR TBI TREATMENT
6.4.2 ABSENCE OF STANDARDIZED TREATMENT PROTOCOLS IN TRAUMATIC BRAIN INJURY MANAGEMENT
7 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TREATMENT
7.1 OVERVIEW
7.2 SURGERY
7.2.1 BRAIN BLEEDING TREATMENT
7.2.2 REHABILITATION
7.2.3 CLOTTED BLOOD REMOVAL
7.2.4 WINDOW OPENING IN SKULL
7.2.5 REPAIRING SKULL FRACTURES
7.3 IMMEDIATE EMERGENCY CARE
7.4 MEDICATIONS
7.4.1 DIURETICS
7.4.2 ANTI-SEIZURE DRUGS (ANTI-CONVULSANT)
7.4.3 ANALGESIC
7.4.4 COMA-INDUCING DRUGS
7.4.5 ANTI-DEPRESSANTS
7.4.6 ANTI-ANXIETY AGENT
7.4.7 ANTI-PSYCHOTICS
7.4.8 ANTI-COAGULANTS
7.4.9 OTHERS
7.4.9.1 Parenteral
7.4.9.2 Oral
7.4.9.3 Others
8 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY PATIENT AGE
8.1 OVERVIEW
8.2 CHILDREN
8.3 TEENAGER
8.4 ELDER
9 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY GENDER
9.1 OVERVIEW
9.2 MALE
9.3 FEMALE
10 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET, CAUSE OF INJURY
10.1 OVERVIEW
10.2 FALLS
10.3 MOTOR VEHICLE TRAFFIC
10.4 SPORTS
10.5 OTHERS
11 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY END USER
11.1 OVERVIEW
11.2 HOSPITALS
11.3 NEUROLOGY CLINICS
11.4 INDEPENDENT PHARMACIES
11.5 OTHERS
12 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY REGION
12.1 EUROPE
12.1.1 GERMANY
12.1.2 U.K.
12.1.3 FRANCE
12.1.4 ITALY
12.1.5 SPAIN
12.1.6 RUSSIA
12.1.7 NETHERLANDS
12.1.8 SWITZERLAND
12.1.9 TURKEY
12.1.10 BELGIUM
12.1.11 REST OF EUROPE
13 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET: COMPANY LANDSCAPE
13.1 COMPANY SHARE ANALYSIS: GLOBAL
14 SWOT ANALYSIS
15 COMPANY PROFILES
15.1 PFIZER INC.
15.1.1 COMPANY SNAPSHOT
15.1.2 REVENUE ANALYSIS
15.1.3 COMPANY SHARE ANALYSIS
15.1.4 PRODUCT PORTFOLIO
15.1.5 RECENT DEVELOPMENT
15.2 TEVA PHARMACEUTICALS USA, INC.
15.2.1 COMPANY SNAPSHOT
15.2.2 REVENUE ANALYSIS
15.2.3 COMPANY SHARE ANALYSIS
15.2.4 PRODUCT PORTFOLIO
15.2.5 RECENT DEVELOPMENT/NEWS
15.3 FRESENIUS SE & CO. KGAA (FRESENIUS KABI AG)
15.3.1 COMPANY SNAPSHOT
15.3.2 REVENUE ANALYSIS
15.3.3 COMPANY SHARE ANALYSIS
15.3.4 PRODUCT PORTFOLIO
15.3.5 RECENT DEVELOPMENT
15.4 VIATRIS INC.
15.4.1 COMPANY SNAPSHOT
15.4.2 REVENUE ANALYSIS
15.4.3 COMPANY SHARE ANALYSIS
15.4.4 PRODUCT PORTFOLIO
15.4.5 RECENT DEVELOPMENT
15.5 AMNEAL PHARMACEUTICALS LLC
15.5.1 COMPANY SNAPSHOT
15.5.2 REVENUE ANALYSIS
15.5.3 COMPANY SHARE ANALYSIS
15.5.4 PRODUCT PORTFOLIO
15.5.5 RECENT DEVELOPMENT
15.6 ADVACARE PHARMA
15.6.1 COMPANY SNAPSHOT
15.6.2 PRODUCT PORTFOLIO
15.6.3 RECENT DEVELOPMENT
15.7 AUROBINDO PHARMA LIMITED
15.7.1 COMPANY SNAPSHOT
15.7.2 REVENUE ANALYSIS
15.7.3 PRODUCT PORTFOLIO
15.7.4 RECENT DEVELOPMENT
15.8 ALEMBIC PHARMACEUTICALS LIMITED
15.8.1 COMPANY SNAPSHOT
15.8.2 REVENUE ANALYSIS
15.8.3 PRODUCT PORTFOLIO
15.8.4 RECENT DEVELOPMENT
15.9 B. BRAUN SE
15.9.1 COMPANY SNAPSHOT
15.9.2 PRODUCT PORTFOLIO
15.9.3 RECENT UPDATES
15.1 DR. REDDY’S LABORATORIES LTD.
15.10.1 COMPANY SNAPSHOT
15.10.2 REVENUE ANALYSIS
15.10.3 PIPELINE PRODUCT PORTFOLIO
15.10.4 RECENT DEVELOPMENT
15.11 ICU MEDICAL, INC.
15.11.1 COMPANY SNAPSHOT
15.11.2 REVENUE ANALYSIS
15.11.3 PRODUCT PORTFOLIO
15.11.4 RECENT DEVELOPMENT/NEWS
15.12 HIKMA PHARMACEUTICALS PLC
15.12.1 COMPANY SNAPSHOT
15.12.2 REVENUE ANALYSIS
15.12.3 PIPELINE PRODUCT PORTFOLIO
15.12.4 RECENT DEVELOPMENT
15.13 JEDUX PARENTERAL PRIVATE LIMITED
15.13.1 COMPANY SNAPSHOT
15.13.2 PRODUCT PORTFOLIO
15.13.3 RECENT DEVELOPMENT
15.14 LUPIN
15.14.1 COMPANY SNAPSHOT
15.14.2 REVENUE ANALYSIS
15.14.3 PRODUCT PORTFOLIO
15.14.4 RECENT DEVELOPMENT
15.15 MERZ THERAPEUTICS
15.15.1 COMPANY SNAPSHOT
15.15.2 PIPELINE PRODUCT PORTFOLIO
15.15.3 RECENT DEVELOPMENT
15.16 MAXZIMAA
15.16.1 COMPANY SNAPSHOT
15.16.2 PIPELINE PRODUCT PORTFOLIO
15.16.3 RECENT DEVELOPMENT
15.17 SUN PHARMACEUTICAL INDUSTRIES LTD.
15.17.1 COMPANY SNAPSHOT
15.17.2 REVENUE ANALYSIS
15.17.3 PRODUCT PORTFOLIO
15.17.4 RECENT DEVELOPMENT
15.18 SWISS PHARMA NIGERIA LIMITED
15.18.1 COMPANY SNAPSHOT
15.18.2 PRODUCT PORTFOLIO
15.18.3 RECENT DEVELOPMENT
15.19 SAGENT
15.19.1 COMPANY SNAPSHOT
15.19.2 PRODUCT PORTFOLIO
15.19.3 RECENT DEVELOPMENT
16 QUESTIONNAIRE
17 RELATED REPORTS
Liste des tableaux
TABLE 1 NON-INVASIVE THERAPEUTIC APPROACHES’ EFFICIENCY IN DIFFERENT PHASES OF TBI
TABLE 2 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 3 EUROPE SURGERY IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 4 EUROPE SURGERY IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 5 EUROPE IMMEDIATE EMERGENCY CARE IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 6 EUROPE MEDICATIONS IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 7 EUROPE MEDICATIONS IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 8 EUROPE MEDICATIONS IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 9 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY PATIENT AGE, 2018-2032 (USD THOUSAND)
TABLE 10 EUROPE CHILDREN IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 11 EUROPE TEENAGER IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 12 EUROPE ELDER IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 13 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 14 EUROPE MALE IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 15 EUROPE FEMALE IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 16 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY CAUSE OF INJURY, 2018-2032 (USD THOUSAND)
TABLE 17 EUROPE FALLS IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 18 EUROPE MOTOR VEHICLE TRAFFIC IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 19 EUROPE SPORTS IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 20 EUROPE OTHERS IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 21 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 22 EUROPE HOSPITALS IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 23 EUROPE NEUROLOGY CLINICS IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 24 EUROPE INDEPENDENT PHARMACIES IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 25 EUROPE OTHERS IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 26 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY COUNTRY, 2018-2032 (USD THOUSAND)
TABLE 27 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 28 EUROPE SURGERY IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 29 EUROPE MEDICATIONS IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 30 EUROPE MEDICATIONS IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 31 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY PATIENT AGE, 2018-2032 (USD THOUSAND)
TABLE 32 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 33 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY CAUSE OF INJURY, 2018-2032 (USD THOUSAND)
TABLE 34 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY END-USER, 2018-2032 (USD THOUSAND)
TABLE 35 GERMANY TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 36 GERMANY SURGERY IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 37 GERMANY MEDICATIONS IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 38 GERMANY MEDICATIONS IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 39 GERMANY TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY PATIENT AGE, 2018-2032 (USD THOUSAND)
TABLE 40 GERMANY TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 41 GERMANY TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY CAUSE OF INJURY, 2018-2032 (USD THOUSAND)
TABLE 42 GERMANY TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY END-USER, 2018-2032 (USD THOUSAND)
TABLE 43 U.K. TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 44 U.K. SURGERY IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 45 U.K. MEDICATIONS IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 46 U.K. MEDICATIONS IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 47 U.K. TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY PATIENT AGE, 2018-2032 (USD THOUSAND)
TABLE 48 U.K. TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 49 U.K. TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY CAUSE OF INJURY, 2018-2032 (USD THOUSAND)
TABLE 50 U.K. TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY END-USER, 2018-2032 (USD THOUSAND)
TABLE 51 FRANCE TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 52 FRANCE SURGERY IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 53 FRANCE MEDICATIONS IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 54 FRANCE MEDICATIONS IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 55 FRANCE TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY PATIENT AGE, 2018-2032 (USD THOUSAND)
TABLE 56 FRANCE TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 57 FRANCE TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY CAUSE OF INJURY, 2018-2032 (USD THOUSAND)
TABLE 58 FRANCE TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY END-USER, 2018-2032 (USD THOUSAND)
TABLE 59 ITALY TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 60 ITALY SURGERY IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 61 ITALY MEDICATIONS IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 62 ITALY MEDICATIONS IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 63 ITALY TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY PATIENT AGE, 2018-2032 (USD THOUSAND)
TABLE 64 ITALY TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 65 ITALY TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY CAUSE OF INJURY, 2018-2032 (USD THOUSAND)
TABLE 66 ITALY TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY END-USER, 2018-2032 (USD THOUSAND)
TABLE 67 SPAIN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 68 SPAIN SURGERY IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 69 SPAIN MEDICATIONS IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 70 SPAIN MEDICATIONS IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 71 SPAIN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY PATIENT AGE, 2018-2032 (USD THOUSAND)
TABLE 72 SPAIN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 73 SPAIN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY CAUSE OF INJURY, 2018-2032 (USD THOUSAND)
TABLE 74 SPAIN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY END-USER, 2018-2032 (USD THOUSAND)
TABLE 75 RUSSIA TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 76 RUSSIA SURGERY IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 77 RUSSIA MEDICATIONS IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 78 RUSSIA MEDICATIONS IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 79 RUSSIA TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY PATIENT AGE, 2018-2032 (USD THOUSAND)
TABLE 80 RUSSIA TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 81 RUSSIA TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY CAUSE OF INJURY, 2018-2032 (USD THOUSAND)
TABLE 82 RUSSIA TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY END-USER, 2018-2032 (USD THOUSAND)
TABLE 83 NETHERLANDS TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 84 NETHERLANDS SURGERY IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 85 NETHERLANDS MEDICATIONS IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 86 NETHERLANDS MEDICATIONS IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 87 NETHERLANDS TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY PATIENT AGE, 2018-2032 (USD THOUSAND)
TABLE 88 NETHERLANDS TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 89 NETHERLANDS TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY CAUSE OF INJURY, 2018-2032 (USD THOUSAND)
TABLE 90 NETHERLANDS TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY END-USER, 2018-2032 (USD THOUSAND)
TABLE 91 SWITZERLAND TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 92 SWITZERLAND SURGERY IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 93 SWITZERLAND MEDICATIONS IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 94 SWITZERLAND MEDICATIONS IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 95 SWITZERLAND TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY PATIENT AGE, 2018-2032 (USD THOUSAND)
TABLE 96 SWITZERLAND TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 97 SWITZERLAND TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY CAUSE OF INJURY, 2018-2032 (USD THOUSAND)
TABLE 98 SWITZERLAND TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY END-USER, 2018-2032 (USD THOUSAND)
TABLE 99 TURKEY TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 100 TURKEY SURGERY IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 101 TURKEY MEDICATIONS IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 102 TURKEY MEDICATIONS IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 103 TURKEY TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY PATIENT AGE, 2018-2032 (USD THOUSAND)
TABLE 104 TURKEY TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 105 TURKEY TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY CAUSE OF INJURY, 2018-2032 (USD THOUSAND)
TABLE 106 TURKEY TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY END-USER, 2018-2032 (USD THOUSAND)
TABLE 107 BELGIUM TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 108 BELGIUM SURGERY IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 109 BELGIUM MEDICATIONS IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 110 BELGIUM MEDICATIONS IN TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 111 BELGIUM TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY PATIENT AGE, 2018-2032 (USD THOUSAND)
TABLE 112 BELGIUM TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 113 BELGIUM TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY CAUSE OF INJURY, 2018-2032 (USD THOUSAND)
TABLE 114 BELGIUM TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY END-USER, 2018-2032 (USD THOUSAND)
TABLE 115 REST OF EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET, BY TREATMENT, 2018-2032 (USD THOUSAND)
Liste des figures
FIGURE 1 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET: SEGMENTATION
FIGURE 2 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET: DATA TRIANGULATION
FIGURE 3 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET: DROC ANALYSIS
FIGURE 4 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET: EUROPE VS REGIONAL MARKET ANALYSIS
FIGURE 5 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET: DBMR MARKET POSITION GRID
FIGURE 8 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET: MARKET END-USER COVERAGE GRID
FIGURE 9 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET: SEGMENTATION
FIGURE 11 INCREASING INCIDENCE OF TRAUMATIC BRAIN INJURY (TBI) IS EXPECTED TO DRIVE THE EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET GROWTH IN THE FORECAST PERIOD OF 2025 TO 2032
FIGURE 12 SURGERY SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET IN THE FORECAST PERIOD OF 2025 & 2032
FIGURE 13 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET: EXECUTIVE SUMMARY
FIGURE 14 STRATEGIC DECISIONS
FIGURE 15 DROC ANALYSIS
FIGURE 16 TBI-RELATED DEATHS FROM 2018 TO 2024
FIGURE 17 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET: BY TREATMENT, 2024
FIGURE 18 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET: BY TREATMENT, 2025 TO 2032 (USD THOUSAND)
FIGURE 19 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET: BY TREATMENT, CAGR (2025- 2032)
FIGURE 20 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET: BY TREATMENT, LIFELINE CURVE
FIGURE 21 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET: BY PATIENT AGE, 2024
FIGURE 22 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET: BY PATIENT AGE, 2025 TO 2032 (USD THOUSAND)
FIGURE 23 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET: BY PATIENT AGE, CAGR (2025- 2032)
FIGURE 24 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET: BY PATIENT AGE, LIFELINE CURVE
FIGURE 25 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET: BY GENDER, 2024
FIGURE 26 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET: BY GENDER, 2025-2032 (USD THOUSAND)
FIGURE 27 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET: BY GENDER CAGR (2025-2032)
FIGURE 28 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET: BY GENDER, LIFELINE CURVE
FIGURE 29 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET: BY CAUSE OF INJURY, 2024
FIGURE 30 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET: BY CAUSE OF INJURY, 2025-2032 (USD THOUSAND)
FIGURE 31 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET: BY CAUSE OF INJURY, CAGR (2025-2032)
FIGURE 32 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET: BY CAUSE OF INJURY, LIFELINE CURVE
FIGURE 33 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET: BY END USER, 2024
FIGURE 34 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET: BY END USER, 2025-2032 (USD THOUSAND)
FIGURE 35 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET: BY END USER, CAGR (2025-2032)
FIGURE 36 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET: BY END USER, LIFELINE CURVE
FIGURE 37 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET: SNAPSHOT (2024)
FIGURE 38 EUROPE TRAUMATIC BRAIN INJURY TREATMENT MARKET: COMPANY SHARE 2024 (%)

Méthodologie de recherche
La collecte de données et l'analyse de l'année de base sont effectuées à l'aide de modules de collecte de données avec des échantillons de grande taille. L'étape consiste à obtenir des informations sur le marché ou des données connexes via diverses sources et stratégies. Elle comprend l'examen et la planification à l'avance de toutes les données acquises dans le passé. Elle englobe également l'examen des incohérences d'informations observées dans différentes sources d'informations. Les données de marché sont analysées et estimées à l'aide de modèles statistiques et cohérents de marché. De plus, l'analyse des parts de marché et l'analyse des tendances clés sont les principaux facteurs de succès du rapport de marché. Pour en savoir plus, veuillez demander un appel d'analyste ou déposer votre demande.
La méthodologie de recherche clé utilisée par l'équipe de recherche DBMR est la triangulation des données qui implique l'exploration de données, l'analyse de l'impact des variables de données sur le marché et la validation primaire (expert du secteur). Les modèles de données incluent la grille de positionnement des fournisseurs, l'analyse de la chronologie du marché, l'aperçu et le guide du marché, la grille de positionnement des entreprises, l'analyse des brevets, l'analyse des prix, l'analyse des parts de marché des entreprises, les normes de mesure, l'analyse globale par rapport à l'analyse régionale et des parts des fournisseurs. Pour en savoir plus sur la méthodologie de recherche, envoyez une demande pour parler à nos experts du secteur.
Personnalisation disponible
Data Bridge Market Research est un leader de la recherche formative avancée. Nous sommes fiers de fournir à nos clients existants et nouveaux des données et des analyses qui correspondent à leurs objectifs. Le rapport peut être personnalisé pour inclure une analyse des tendances des prix des marques cibles, une compréhension du marché pour d'autres pays (demandez la liste des pays), des données sur les résultats des essais cliniques, une revue de la littérature, une analyse du marché des produits remis à neuf et de la base de produits. L'analyse du marché des concurrents cibles peut être analysée à partir d'une analyse basée sur la technologie jusqu'à des stratégies de portefeuille de marché. Nous pouvons ajouter autant de concurrents que vous le souhaitez, dans le format et le style de données que vous recherchez. Notre équipe d'analystes peut également vous fournir des données sous forme de fichiers Excel bruts, de tableaux croisés dynamiques (Fact book) ou peut vous aider à créer des présentations à partir des ensembles de données disponibles dans le rapport.