Global Achondroplasia Market
Taille du marché en milliards USD
TCAC :
% 
 USD
68.73 Million
USD
828.34 Million
2021
2029
USD
68.73 Million
USD
828.34 Million
2021
2029
| 2022 –2029 | |
| USD 68.73 Million | |
| USD 828.34 Million | |
|
|
|
|
Global Achondroplasia Market, By Treatment (Growth Hormone Therapy, Surgery, Supportive Therapy, Others), Route of Administration (Oral, Parenteral), End-Users (Hospitals, Homecare, Speciality Centres, Others), Distribution Channel (Hospital Pharmacy, Online Pharmacy, Retail Pharmacy) – Industry Trends and Forecast to 2029.
Achondroplasia Market Analysis and Size
The global achondroplasia market is expected to witness significant growth during the forecast period. Achondroplasia is the most common form of dwarfism. The condition is known to affect 1 in 15,000 to 40,000 newborns. The severe complications or risk factors that are associated with this disease are breathing difficulties such as obesity, sleep apnea, spinal stenosis, and recurrent ear infections. This disorder does not normally affect intelligence. Mutations in the FGFR3 gene cause achondroplasia. COVID-19 also had a major impact on the market growth.
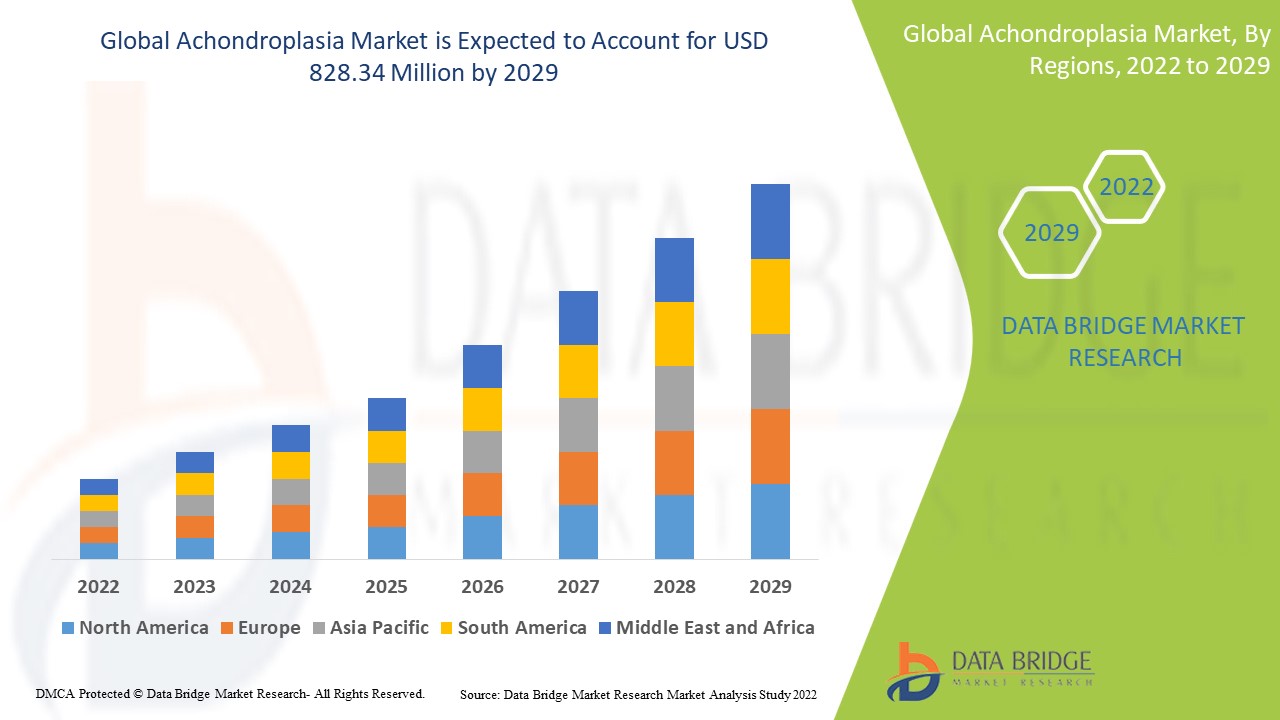
Data Bridge Market Research analyses a growth rate in the global achondroplasia market in the forecast period 2022-2029. The expected CAGR of global achondroplasia market is tend to be around 36.5% in the mentioned forecast period. The market was valued at USD 68.73 million in 2021, and it would grow upto USD 828.34 million by 2029. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Achondroplasia Market Scope and Segmentation
|
Report Metric |
Details |
|
Forecast Period |
2022 to 2029 |
|
Base Year |
2021 |
|
Historic Years |
2020 (Customizable to 2014 - 2019) |
|
Quantitative Units |
Revenue in USD Million, Volumes in Units, Pricing in USD |
|
Segments Covered |
Treatment (Growth Hormone Therapy, Surgery, Supportive Therapy, Others), Route of Administration (Oral, Parenteral), End-Users (Hospitals, Homecare, Speciality Centres, Others), Distribution Channel (Hospital Pharmacy, Online Pharmacy, Retail Pharmacy) |
|
Countries Covered |
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America |
|
Market Players Covered |
Teva Pharmaceutical Industries Ltd (Israel), Mylan N.V (U.S.), Johnsons & Johnsons Services Inc (U.S.), F. Hoffman-La Roche Ltd. (Switzerland), Lilly (U.S.), Merck & Co., Inc. (U.S.), Aurobindo Pharma (India), Bristol-Myers Squibb Company (U.S.), GSK plc (U.K.), Ascendis Pharma A/S (Denmark), OCUGEN, INC (U.S.), RIBOMIC (Japan), QED Therapeutics, Inc (U.S.) |
|
Market Opportunities |
|
Market Definition
Arachnoiditis is also known as achondroplasia dwarfism, which is the bone growth disorder and the most common form of short-limbed dwarfism. It is largely characterized by short stature and short limbs. The genetic defect can be passed from parent to child. Although, in around 80% of instances, achondroplasia is caused by a spontaneous mutation that originates in the developing embryo. It is of great importance to the healthcare sector and thus is expected to rise high in the forecast period
Global Achondroplasia Market Dynamics
Drivers
- Increased Incidence of Achondroplasia
The growing prevalence of achondroplasia is projected to drive the market growth during the forecast period. For instance, as per the data published by the European Medicines Agency in June 2021, it is projected that 350 children are born with achondroplasia each year in Europe. This boosts the market growth.
- Increasing Drug Approvals and Launches
The increasing number of drug approvals and launches by major market players is expected to drive market growth. For example, Ascendis Pharma A/S announced on January 13, 2022, that the European Commission (EC) approved TransCon hGH as a once-weekly subcutaneous injection to treat paediatric patients experiencing growth failure due to insufficient endogenous growth hormone secretion or growth hormone deficiency. TransCon hGH contains Somatropin, which delivers unmodified Somatropin (hGH) to the body in therapeutic quantities.
Opportunities
- Higher Adoption in Oral Route of Administration
The oral segment is anticipated to dominate the segment growth during the forecast period, due to the increasing R&D activities for the oral administration of drugs for the treatment of achondroplasia. For instance, as per an article published in PLOS One in April 2020, the drug meclizine hydrochloride inhibited fibroblast growth factor receptor 3 (FGFR3) signaling in numerous chondrocytic cells and promoted longitudinal bone growth in mouse model of achondroplasia. The results stated that oral medicine administration once a day or twice a day was potentially safe and well tolerated with no adverse events in children with achondroplasia. This hampers the market growth.
- Rise in Awareness and Increased Research Activities
Increase in awareness about timely diagnosis of congenital disorders is anticipated to boost the global achondroplasia market during the forecast period. An increase in cases of achondroplasia results in a rise in R&D by companies and research institutes in clinical trials to manufacture novel therapeutics. Thus, these factors drive the market growth.
Restraints/Challenges
- High Cost
The high cost associated with the treatment process of achondroplasia are considered to restrain the market growth. For instance, the growth hormone treatment therapy can be expensive, ranging from US$ 10,000 to US$ 60,000 per year for growth hormone treatment alone. Additionally, the list price of VOXZOGO in France under the ATU (Temporary Authorization for Use) process is 712€ (US$ 837.3 ) per vial.
- Lack of Skilled Professionals
Lack of trained professionals who are not aware of the appropriate treatment methods for this disease could restrain the growth of the global achondroplasia market over a forecast period.
This global achondroplasia market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the global achondroplasia market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
COVID-19 Impact on Global Achondroplasia Market
During the pandemic, factors such as investments in R&D of novel growth hormone products targeting achondroplasia, and funding by various companies operating in the research and development sector, were postponed, which is expected to stymie global achondroplasia market growth.
Furthermore, the COVID-19 pandemic has had an impact on ongoing clinical trials for achondroplasia, which is expected to impede the global achondroplasia market during the forecast period due to product clinical trials that have been delayed. For instance, Ascendis Pharma A/S 2019 annual report stated that the pandemic had a potential impact on the conduct of clinical trials of investigational drug candidates.
Global Achondroplasia Market Scope
The global achondroplasia market is segmented on the basis of treatment, route of administration, distribution channel and end-user. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Treatment
- Growth Hormone Therapy
- Surgery
- Supportive Therapy
- Others
Route of Administration
- Oral
- Parenteral
End-Users
- Hospitals
- Homecare
- Speciality Centres
- Others
Distribution Channel
- Hospital Pharmacy
- Online Pharmacy
- Retail Pharmacy
Achondroplasia Market Regional Analysis/Insights
The global achondroplasia market is analysed and market size insights and trends are provided by treatment, route of administration, distribution channel and end-user as referenced above.
The major countries covered in the global achondroplasia market report are the U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
North America is expected to have the highest market growth due to the high-income of the countries and advanced healthcare facilities.
Asia-Pacific dominates the market due to the increase’s cases of cardiovascular diseases and a rapidly aging population.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Competitive Landscape and Global Achondroplasia Market Share Analysis
The global achondroplasia market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to global achondroplasia market.
Key players operating in the global achondroplasia market include:
- Teva Pharmaceutical Industries Ltd (Israel)
- Mylan N.V (U.S.)
- Johnsons & Johnsons Services Inc (U.S.)
- F. Hoffman-La Roche Ltd. (Switzerland)
- Lilly (U.S.)
- Merck & Co., Inc. (U.S.)
- Aurobindo Pharma (India)
- Bristol-Myers Squibb Company (U.S.)
- GSK plc (U.K.)
- Ascendis Pharma A/S (Denmark)
- OCUGEN, INC (U.S.)
- RIBOMIC (Japan)
- QED Therapeutics, Inc (U.S.)
SKU-
Accédez en ligne au rapport sur le premier cloud mondial de veille économique
- Tableau de bord d'analyse de données interactif
- Tableau de bord d'analyse d'entreprise pour les opportunités à fort potentiel de croissance
- Accès d'analyste de recherche pour la personnalisation et les requêtes
- Analyse de la concurrence avec tableau de bord interactif
- Dernières actualités, mises à jour et analyse des tendances
- Exploitez la puissance de l'analyse comparative pour un suivi complet de la concurrence
Table des matières
1. INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL ACHONDROPLASIA MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2. MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE GLOBAL ACHONDROPLASIA MARKET SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 EPIDEMIOLOGY
2.2.12 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.13 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 GLOBAL ACHONDROPLASIA MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3. MARKET OVERVIEW
3.1 DRIVERS
3.2 RESTRAINTS
3.3 OPPORTUNITIES
3.4 CHALLENGES
4. EXECUTIVE SUMMARY
5. PREMIUM INSIGHTS
5.1 PESTEL ANALYSIS
5.2 PORTER’S FIVE FORCES MODEL
6. INDUSTRY INSIGHTS
6.1 MICRO AND MACRO ECONOMIC FACTORS
6.2 PENETRATION AND GROWTH PROSPECT MAPPING
6.3 KEY PRICING STRATEGIES
6.4 INTERVIEWS WITH SPECIALIST
6.5 ANALYIS AND RECOMMENDATION
7. INTELLECTUAL PROPERTY (IP) PORTFOLIO
7.1 PATENT QUALITY AND STRENGTH
7.2 PATENT FAMILIES
7.3 LICENSING AND COLLABORATIONS
7.4 COMPETITIVE LANDSCAPE
7.5 IP STRATEGY AND MANAGEMENT
7.6 OTHER
8. COST ANALYSIS BREAKDOWN
9. TECHNONLOGY ROADMAP
10. INNOVATION TRACKER AND STRATEGIC ANALYSIS
10.1 MAJOR DEALS AND STRATEGIC ALLIANCES ANALYSIS
10.1.1 JOINT VENTURES
10.1.2 MERGERS AND ACQUISITIONS
10.1.3 LICENSING AND PARTNERSHIP
10.1.4 TECHNOLOGY COLLABORATIONS
10.1.5 STRATEGIC DIVESTMENTS
10.2 NUMBER OF PRODUCTS IN DEVELOPMENT
10.3 STAGE OF DEVELOPMENT
10.4 TIMELINES AND MILESTONES
10.5 INNOVATION STRATEGIES AND METHODOLOGIES
10.6 RISK ASSESSMENT AND MITIGATION
10.7 MERGERS AND ACQUISITIONS
10.8 FUTURE OUTLOOK
11. EPIDEMIOLOGY
11.1 INCIDENCE OF ALL BY GENDER
11.2 TREATMENT RATE
11.3 MORTALITY RATE
11.4 DRUG ADHERENCE AND THERAPY SWITCH MODEL
11.5 PATIENT TREATMENT SUCCESS RATES
11.6 RISK FACTOR
12. REGULATORY COMPLIANCE
12.1 REGULATORY AUTHORITIES
12.2 REGULATORY CLASSIFICATIONS
12.2.1 CLASS I
12.2.2 CLASS II
12.2.3 CLASS III
12.3 REGULATORY SUBMISSIONS
12.4 INTERNATIONAL HARMONIZATION
12.5 COMPLIANCE AND QUALITY MANAGEMENT SYSTEMS
12.6 REGULATORY CHALLENGES AND STRATEGIES
13. PIPELINE ANALYSIS
13.1 CLINICAL TRIALS AND PHASE ANALYSIS
13.2 DRUG THERAPY PIPELINE
13.3 PHASE III CANDIDATES
13.4 PHASE II CANDIDATES
13.5 PHASE I CANDIDATES
13.6 OTHERS (PRE-CLINICAL AND RESEARCH)
14. REIMBURSEMENT FRAMEWORK
15. OPPUTUNITY MAP ANALYSIS
16. VALUE CHAIN ANALYSIS
17. HEALTHCARE ECONOMY
17.1 HEALTHCARE EXPENDITURE
17.2 CAPITAL EXPENDITURE
17.3 CAPEX TRENDS
17.4 CAPEX ALLOCATION
17.5 FUNDING SOURCES
17.6 INDUSTRY BENCHMARKS
17.7 GDP RATION IN OVERALL GDP
17.8 HEALTHCARE SYSTEM STRUCTURE
17.9 GOVERNMENT POLICIES
17.10 ECONOMIC DEVELOPMENT
18. GLOBAL ACHONDROPLASIA MARKET, BY TYPE
18.1 OVERVIEW
18.2 INHERITED
18.2.1 HOMOZYGOUS
18.2.2 HETEROZYGOUS
18.3 NON-INHERITED
19. GLOBAL ACHONDROPLASIA MARKET, BY TREATMENT
(NOTE: MARKET VALUE, VOLUME AND ASP ANALYSIS WOULD BE PROVIDED FOR ALL SEGMENTS AND SUBSEGMENTS OF INDICATION)
19.1 OVERVIEW
19.2 HORMONE THERAPY
19.2.1 MARKETED
19.2.1.1. BY DRUG
19.2.1.1.1. VOSORITIDE
19.2.1.1.1.1 0.4MG/VIAL
19.2.1.1.1.2 0.56MG/VIAL
19.2.1.1.1.3 1.2MG/VIAL
19.2.1.1.2. GENOTROPIN MINIQUICK
19.2.1.1.2.1 0.2MG
19.2.1.1.2.2 0.4MG
19.2.1.1.2.3 0.6MG
19.2.1.1.2.4 0.8MG
19.2.1.1.2.5 1MG
19.2.1.1.2.6 1.2MG
19.2.1.1.2.7 1.4MG
19.2.1.1.2.8 1.6MG
19.2.1.1.2.9 1.8MG
19.2.1.1.2.10 OTHERS
19.2.1.1.3. GENOTROPIN
19.2.1.1.3.1 5MG
19.2.1.1.3.2 12MG
19.2.1.1.4. HUMATROPE
19.2.1.1.4.1 5MG
19.2.1.1.4.2 6MG
19.2.1.1.4.3 12MG
19.2.1.1.4.4 OTHERS
19.2.1.1.5. SAIZEN
19.2.1.1.5.1 5MG
19.2.1.1.5.2 8.8MG
19.2.1.1.6. SEROSTIM
19.2.1.1.6.1 4MG
19.2.1.1.6.2 5MG
19.2.1.1.6.3 6MG
19.2.1.1.7. ZOMACTON
19.2.1.1.7.1 5MG
19.2.1.1.7.2 10MG
19.2.1.1.8. NORDITROPIN FLEXPRO
19.2.1.1.8.1 5MG/1.5ML
19.2.1.1.8.2 10MG/1.5ML
19.2.1.1.8.3 15MG/1.5ML
19.2.1.1.8.4 30MG/3ML
19.2.1.1.9. OMNITROPE
19.2.1.1.9.1 5MG/1.5ML
19.2.1.1.9.2 10MG/1.5ML
19.2.1.1.9.3 OTHERS
19.2.1.1.10. NUTROPIN
19.2.1.1.10.1 NUTROPIN AQ NUSPIN 20
19.2.1.1.10.2 NUTROPIN AQ NUSPIN 10
19.2.1.1.10.3 NUTROPIN AQ NUSPIN 5
19.2.1.1.11. ZORBTIVE
19.2.1.1.12. OTHERS
19.2.1.2. BY DRUG TYPE
19.2.1.2.1. BRANDED
19.2.1.2.2. GENERICS
19.2.1.3. BY AGE GROUP
19.2.1.3.1. PEDIATRIC
19.2.1.3.2. ADULT
19.2.1.3.3. GERIATRIC
19.2.1.4. OTHERS
19.2.2 PIPELINE
19.2.2.1. FGFR1-3
19.2.2.2. TRANSCON CNP
19.2.2.3. INFIGRATINIB (BBP-831/BGJ398)
19.2.2.4. SAR-442501
19.2.2.5. RBM-007
19.2.2.6. SAR442501
19.2.2.7. OTHERS
19.3 SURGERY
19.3.1 SPINE
19.3.1.1. SPINAL CANAL STENOSIS TREATMENT
19.3.1.2. THORACOLUMBAR KYPHOSIS TREATMENT
19.3.1.3. GENU VARUM CORRECTION
19.3.1.4. FORAMEN MAGNUM DECOMPRESSION
19.3.1.5. OTHERS
19.3.2 EXTREMITY
19.3.2.1. TIBIAL +/- FEMUR OSTEOTOMIES
19.3.2.2. LOWER LIMB LENGTHENING
19.3.2.3. UPPER EXTREMITY LENGTHENING
19.4 SUPPORTIVE THERAPY
19.4.1 PHYSICAL THERAPY
19.4.2 OCCUPATIONAL THERAPY
19.4.3 REHABILITATION PHYSICIAN
19.4.4 PSYCHOSOCIAL SUPPORT
19.4.5 NUTRITIONAL SUPPORT
19.4.6 OTHERS
19.5 EMERGING TREATMENT (POTENTIAL CANDIDATES)
19.5.1 DRUGS TARGETING THE FGFR3 LIGANDS
19.5.1.1. FIBROBLAST GROWTH FACTOR 2 APTAMER (RBM-007)
19.5.1.2. SOLUBLE FGFR3 DECOY (TA-46)
19.5.1.3. OTHERS
19.5.2 DRUGS TARGETING THE FGFR3 AND DOWNSTREAM SIGNALLING
19.5.2.1. ANTI-FGFR3 ANTIBODY (B-701)
19.5.2.2. TYROSINE KINASE INHIBITION (BGJ398)
19.5.2.3. MECLOZINE/MECLIZINE
19.5.3 DRUGS TARGETING THE CNP RECEPTOR NPR-B
19.5.3.1. CNP ANALOGUE VOSORITIDE (BMN111)
19.5.3.2. HUMAN CNP (CNP-53)
19.5.3.3. OTHERS
19.6 OTHERS
20. GLOBAL ACHONDROPLASIA MARKET, BY ROUTE OF ADMINISTRATION
20.1 OVERVIEW
20.2 ORAL
20.3 PARENTERAL
20.3.1 SUBCUTANEOUS
20.3.2 INTRAMUCSULAR
20.3.3 OTHERS
20.4 OTHERS
21. GLOBAL ACHONDROPLASIA MARKET, BY GENDER
21.1 OVERVIEW
21.2 MALE
21.2.1 PEDIATRIC
21.2.2 ADULT
21.2.3 GERIATRIC
21.3 FEMALE
21.3.1 PEDIATRIC
21.3.2 ADULT
21.3.3 GERIATRIC
22. GLOBAL ACHONDROPLASIA MARKET, BY POPULATION TYPE
22.1 OVERVIEW
22.2 PEDIATRIC
22.3 ADULT
22.4 GERIATRIC
23. GLOBAL ACHONDROPLASIA MARKET, BY INDICATION
23.1 OVERVIEW
23.2 BOWED LEGS
23.3 CENTRAL SLEEP APNEA
23.4 OBSTRUCTIVE SLEEP APNEA
23.5 HEARING IMPAIRMENT
23.6 PSEUDOCLAUDICATION
23.7 RESTRICTIVE PULMONARY DISEASE
23.8 DISORDERS OF CRANIAL VOLUME AND SHAPE
23.9 OTHERS
24. GLOBAL ACHONDROPLASIA MARKET, BY END USER
24.1 OVERVIEW
24.2 HOSPITALS
24.2.1 PRIVATE
24.2.2 PUBLIC
24.3 SPECIALTY CLINICS
24.4 HOME HEALTHCARE
24.5 RESEARCH AND ACADEMIC INSTITUTE
24.6 OTHERS
25. GLOBAL ACHONDROPLASIA MARKET, BY DISTRIBUTION CHANNEL
25.1 OVERVIEW
25.2 DIRECT TENDER
25.3 RETAIL SALES
25.3.1 HOSPITAL PHARMACIES
25.3.2 ONLINE PHARMACY
25.3.3 MEDICINE STORES
25.4 OTHER
26. GLOBAL ACHONDROPLASIA MARKET, SWOT AND DBMR ANALYSIS
27. GLOBAL ACHONDROPLASIA MARKET, COMPANY LANDSCAPE
27.1 COMPANY SHARE ANALYSIS: GLOBAL
27.2 COMPANY SHARE ANALYSIS: NORTH AMERICA
27.3 COMPANY SHARE ANALYSIS: EUROPE
27.4 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
27.5 COMPANY SHARE ANALYSIS: MIDDLE EAST AND AFRICA
27.6 MERGERS & ACQUISITIONS
27.7 NEW PRODUCT DEVELOPMENT & APPROVALS
27.8 EXPANSIONS
27.9 REGULATORY CHANGES
27.10 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
28. GLOBAL ACHONDROPLASIA MARKET, BY REGION
GLOBAL ACHONDROPLASIA MARKET, (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
28.1 NORTH AMERICA
28.1.1 U.S.
28.1.2 CANADA
28.1.3 MEXICO
28.2 EUROPE
28.2.1 GERMANY
28.2.2 U.K.
28.2.3 ITALY
28.2.4 FRANCE
28.2.5 SPAIN
28.2.6 RUSSIA
28.2.7 SWITZERLAND
28.2.8 TURKEY
28.2.9 BELGIUM
28.2.10 NETHERLANDS
28.2.11 DENMARK
28.2.12 SWEDEN
28.2.13 POLAND
28.2.14 NORWAY
28.2.15 FINLAND
28.2.16 REST OF EUROPE
28.3 ASIA-PACIFIC
28.3.1 JAPAN
28.3.2 CHINA
28.3.3 SOUTH KOREA
28.3.4 INDIA
28.3.5 SINGAPORE
28.3.6 THAILAND
28.3.7 INDONESIA
28.3.8 MALAYSIA
28.3.9 PHILIPPINES
28.3.10 AUSTRALIA
28.3.11 NEW ZEALAND
28.3.12 VIETNAM
28.3.13 TAIWAN
28.3.14 REST OF ASIA-PACIFIC
28.4 SOUTH AMERICA
28.4.1 BRAZIL
28.4.2 ARGENTINA
28.4.3 REST OF SOUTH AMERICA
28.5 MIDDLE EAST AND AFRICA
28.5.1 SOUTH AFRICA
28.5.2 EGYPT
28.5.3 BAHRAIN
28.5.4 UNITED ARAB EMIRATES
28.5.5 KUWAIT
28.5.6 OMAN
28.5.7 QATAR
28.5.8 SAUDI ARABIA
28.5.9 REST OF MEA
28.6 KEY PRIMARY INSIGHTS: BY MAJOR COUNTRIES
29. GLOBAL ACHONDROPLASIA MARKET, COMPANY PROFILE
29.1 BIOMARIN PHARMACEUTICAL INC.
29.1.1 COMPANY OVERVIEW
29.1.2 REVENUE ANALYSIS
29.1.3 GEOGRAPHIC PRESENCE
29.1.4 PRODUCT PORTFOLIO
29.1.5 RECENT DEVELOPEMENTS
29.2 BRIDGEBIO INC.
29.2.1 COMPANY OVERVIEW
29.2.2 REVENUE ANALYSIS
29.2.3 GEOGRAPHIC PRESENCE
29.2.4 PRODUCT PORTFOLIO
29.2.5 RECENT DEVELOPEMENTS
29.3 ASCENDIS PHARMA A/S.
29.3.1 COMPANY OVERVIEW
29.3.2 REVENUE ANALYSIS
29.3.3 GEOGRAPHIC PRESENCE
29.3.4 PRODUCT PORTFOLIO
29.3.5 RECENT DEVELOPEMENTS
29.4 SANOFI
29.4.1 COMPANY OVERVIEW
29.4.2 REVENUE ANALYSIS
29.4.3 GEOGRAPHIC PRESENCE
29.4.4 PRODUCT PORTFOLIO
29.4.5 RECENT DEVELOPEMENTS
29.5 TYRA BIOSCIENCES, INC.
29.5.1 COMPANY OVERVIEW
29.5.2 REVENUE ANALYSIS
29.5.3 GEOGRAPHIC PRESENCE
29.5.4 PRODUCT PORTFOLIO
29.5.5 RECENT DEVELOPEMENTS
29.6 PFIZER INC.
29.6.1 COMPANY OVERVIEW
29.6.2 REVENUE ANALYSIS
29.6.3 GEOGRAPHIC PRESENCE
29.6.4 PRODUCT PORTFOLIO
29.6.5 RECENT DEVELOPEMENTS
29.7 RIBOMIC
29.7.1 COMPANY OVERVIEW
29.7.2 REVENUE ANALYSIS
29.7.3 GEOGRAPHIC PRESENCE
29.7.4 PRODUCT PORTFOLIO
29.7.5 RECENT DEVELOPEMENTS
29.8 PHASEBIO PHARMACEUTICALS, INC.
29.8.1 COMPANY OVERVIEW
29.8.2 REVENUE ANALYSIS
29.8.3 GEOGRAPHIC PRESENCE
29.8.4 PRODUCT PORTFOLIO
29.8.5 RECENT DEVELOPEMENTS
29.9 PROLYNX INC
29.9.1 COMPANY OVERVIEW
29.9.2 REVENUE ANALYSIS
29.9.3 GEOGRAPHIC PRESENCE
29.9.4 PRODUCT PORTFOLIO
29.9.5 RECENT DEVELOPEMENTS
29.10 NOVARTIS AG
29.10.1 COMPANY OVERVIEW
29.10.2 REVENUE ANALYSIS
29.10.3 GEOGRAPHIC PRESENCE
29.10.4 PRODUCT PORTFOLIO
29.10.5 RECENT DEVELOPEMENTS
29.11 USV PRIVATE LIMITED
29.11.1 COMPANY OVERVIEW
29.11.2 REVENUE ANALYSIS
29.11.3 GEOGRAPHIC PRESENCE
29.11.4 PRODUCT PORTFOLIO
29.11.5 RECENT DEVELOPEMENTS
29.12 LG CHEM
29.12.1 COMPANY OVERVIEW
29.12.2 REVENUE ANALYSIS
29.12.3 GEOGRAPHIC PRESENCE
29.12.4 PRODUCT PORTFOLIO
29.12.5 RECENT DEVELOPEMENTS
29.13 FERRING
29.13.1 COMPANY OVERVIEW
29.13.2 REVENUE ANALYSIS
29.13.3 GEOGRAPHIC PRESENCE
29.13.4 PRODUCT PORTFOLIO
29.13.5 RECENT DEVELOPEMENTS
29.14 SOMATROPIN BIOPARTNERS GMBH
29.14.1 COMPANY OVERVIEW
29.14.2 REVENUE ANALYSIS
29.14.3 GEOGRAPHIC PRESENCE
29.14.4 PRODUCT PORTFOLIO
29.14.5 RECENT DEVELOPEMENTS
29.15 BIOSIDUS
29.15.1 COMPANY OVERVIEW
29.15.2 REVENUE ANALYSIS
29.15.3 GEOGRAPHIC PRESENCE
29.15.4 PRODUCT PORTFOLIO
29.15.5 RECENT DEVELOPEMENTS
29.16 ANKEBIO CO., LTD
29.16.1 COMPANY OVERVIEW
29.16.2 REVENUE ANALYSIS
29.16.3 GEOGRAPHIC PRESENCE
29.16.4 PRODUCT PORTFOLIO
29.16.5 RECENT DEVELOPEMENTS
29.17 ELI LILLY AND COMPANY
29.17.1 COMPANY OVERVIEW
29.17.2 REVENUE ANALYSIS
29.17.3 GEOGRAPHIC PRESENCE
29.17.4 PRODUCT PORTFOLIO
29.17.5 RECENT DEVELOPEMENTS
29.18 NOVO NORDISK A/S
29.18.1 COMPANY OVERVIEW
29.18.2 REVENUE ANALYSIS
29.18.3 GEOGRAPHIC PRESENCE
29.18.4 PRODUCT PORTFOLIO
29.18.5 RECENT DEVELOPEMENTS
29.19 IPSEN PHARMA
29.19.1 COMPANY OVERVIEW
29.19.2 REVENUE ANALYSIS
29.19.3 GEOGRAPHIC PRESENCE
29.19.4 PRODUCT PORTFOLIO
29.19.5 RECENT DEVELOPEMENTS
29.20 AETERNA ZENTARIS
29.20.1 COMPANY OVERVIEW
29.20.2 REVENUE ANALYSIS
29.20.3 GEOGRAPHIC PRESENCE
29.20.4 PRODUCT PORTFOLIO
29.20.5 RECENT DEVELOPMENTS
29.21 MERCK KGAA
29.21.1 COMPANY OVERVIEW
29.21.2 REVENUE ANALYSIS
29.21.3 GEOGRAPHIC PRESENCE
29.21.4 PRODUCT PORTFOLIO
29.21.5 RECENT DEVELOPMENTS
29.22 GENESCIENCE PHARMACEUTICAL CO., LTD.
29.22.1 COMPANY OVERVIEW
29.22.2 REVENUE ANALYSIS
29.22.3 GEOGRAPHIC PRESENCE
29.22.4 PRODUCT PORTFOLIO
29.22.5 RECENT DEVELOPMENTS
29.23 DONG-A ST
29.23.1 COMPANY OVERVIEW
29.23.2 REVENUE ANALYSIS
29.23.3 GEOGRAPHIC PRESENCE
29.23.4 PRODUCT PORTFOLIO
29.23.5 RECENT DEVELOPMENTS
30. RELATED REPORTS
31. CONCLUSION
32. QUESTIONNAIRE
33. ABOUT DATA BRIDGE MARKET RESEARCH

Méthodologie de recherche
La collecte de données et l'analyse de l'année de base sont effectuées à l'aide de modules de collecte de données avec des échantillons de grande taille. L'étape consiste à obtenir des informations sur le marché ou des données connexes via diverses sources et stratégies. Elle comprend l'examen et la planification à l'avance de toutes les données acquises dans le passé. Elle englobe également l'examen des incohérences d'informations observées dans différentes sources d'informations. Les données de marché sont analysées et estimées à l'aide de modèles statistiques et cohérents de marché. De plus, l'analyse des parts de marché et l'analyse des tendances clés sont les principaux facteurs de succès du rapport de marché. Pour en savoir plus, veuillez demander un appel d'analyste ou déposer votre demande.
La méthodologie de recherche clé utilisée par l'équipe de recherche DBMR est la triangulation des données qui implique l'exploration de données, l'analyse de l'impact des variables de données sur le marché et la validation primaire (expert du secteur). Les modèles de données incluent la grille de positionnement des fournisseurs, l'analyse de la chronologie du marché, l'aperçu et le guide du marché, la grille de positionnement des entreprises, l'analyse des brevets, l'analyse des prix, l'analyse des parts de marché des entreprises, les normes de mesure, l'analyse globale par rapport à l'analyse régionale et des parts des fournisseurs. Pour en savoir plus sur la méthodologie de recherche, envoyez une demande pour parler à nos experts du secteur.
Personnalisation disponible
Data Bridge Market Research est un leader de la recherche formative avancée. Nous sommes fiers de fournir à nos clients existants et nouveaux des données et des analyses qui correspondent à leurs objectifs. Le rapport peut être personnalisé pour inclure une analyse des tendances des prix des marques cibles, une compréhension du marché pour d'autres pays (demandez la liste des pays), des données sur les résultats des essais cliniques, une revue de la littérature, une analyse du marché des produits remis à neuf et de la base de produits. L'analyse du marché des concurrents cibles peut être analysée à partir d'une analyse basée sur la technologie jusqu'à des stratégies de portefeuille de marché. Nous pouvons ajouter autant de concurrents que vous le souhaitez, dans le format et le style de données que vous recherchez. Notre équipe d'analystes peut également vous fournir des données sous forme de fichiers Excel bruts, de tableaux croisés dynamiques (Fact book) ou peut vous aider à créer des présentations à partir des ensembles de données disponibles dans le rapport.













