Global Car Nk Cell Therapy Market
Taille du marché en milliards USD
TCAC :
% 
 USD
346.50 Million
USD
1,004.50 Million
2025
2033
USD
346.50 Million
USD
1,004.50 Million
2025
2033
| 2026 –2033 | |
| USD 346.50 Million | |
| USD 1,004.50 Million | |
|
|
|
|
Global CAR-NK Cell Therapy Market Segmentation By Indication ( Hematological Malignancies, Solid Tumors, and Others), End User ( Hospitals, Specialized Cancer Centers, Research & Academic Institutes, and Others) - Industry Trends and Forecast to 2033
CAR-NK Cell Therapy Market Size
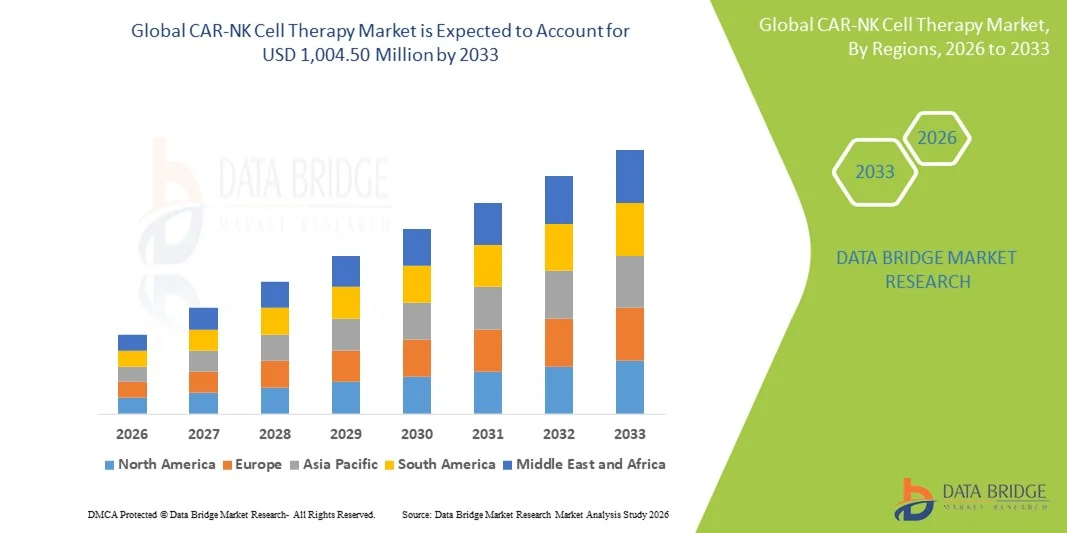
- The global CAR-NK cell therapy market size was valued at USD 346.5 Million in 2025 and is expected to reach USD 1,004.50 Million by 2033, at a CAGR of 14.20% during the forecast period
- The market growth is largely fueled by increasing investments in advanced immunotherapies, rising prevalence of cancer, and continuous technological progress in genetic engineering and cell modification techniques, driving wider adoption of CAR-NK cell therapy across clinical and research settings
- Furthermore, growing demand for safer and more effective cell-based cancer treatments, along with advantages such as lower risk of graft-versus-host disease and off-the-shelf potential, is accelerating the uptake of CAR-NK cell therapy solutions, thereby significantly boosting the market’s growth
CAR-NK Cell Therapy Market Analysis
- CAR-NK cell therapy, an emerging form of adoptive cell immunotherapy, is gaining strong traction in oncology due to its ability to offer targeted anti-tumor activity with improved safety, reduced cytokine release syndrome risk, and off-the-shelf treatment potential compared to CAR-T therapies
- The escalating demand for CAR-NK cell therapy is primarily driven by the rising global cancer burden, growing focus on next-generation immunotherapies, increasing clinical trial activity, and continuous advancements in genetic engineering and cell manufacturing technologies
- North America dominated the CAR-NK Cell Therapy market with the largest revenue share of approximately 38.6% in 2025, supported by strong R&D infrastructure, high oncology research funding, early adoption of advanced cell therapies, and a robust presence of biotechnology and pharmaceutical companies, with the U.S. accounting for the majority of regional revenue
- Asia-Pacific is expected to be the fastest-growing region in the CAR-NK Cell Therapy market during the forecast period, registering a high CAGR, driven by expanding biotechnology sectors, increasing government support for cell therapy research, rising cancer prevalence, and improving clinical trial capabilities in countries such as China, Japan, and South Korea
- The hematological malignancies segment dominated the largest market revenue share of 58.4% in 2025, driven by the high clinical success of CAR-NK therapies in blood cancers such as leukemia, lymphoma, and multiple myeloma
Report Scope and CAR-NK Cell Therapy Market Segmentation
|
Attributes |
CAR-NK Cell Therapy Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
• Nkarta Therapeutics (U.S.) |
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
CAR-NK Cell Therapy Market Trends
Advancement of Allogeneic and Off-the-Shelf CAR-NK Cell Platforms
- A significant and accelerating trend in the global CAR-NK Cell Therapy market is the shift toward allogeneic, off-the-shelf CAR-NK therapies, which offer improved safety profiles, faster manufacturing timelines, and reduced treatment costs compared to autologous CAR-T therapies. CAR-NK cells demonstrate lower risks of cytokine release syndrome (CRS) and graft-versus-host disease (GvHD), making them suitable for broader patient populations
- For instance, in March 2023, Fate Therapeutics reported positive interim clinical data from its FT516 off-the-shelf CAR-NK therapy in patients with relapsed or refractory B-cell lymphoma, demonstrating encouraging safety and anti-tumor activity
- The availability of pre-manufactured CAR-NK cell products significantly reduces treatment initiation time, which is critical for aggressive hematological malignancies. This trend supports scalability and commercial viability of CAR-NK therapies
- Advancements in genetic engineering, including improved viral and non-viral transduction techniques, are enhancing CAR expression and persistence of NK cells
- The use of induced pluripotent stem cells (iPSCs) as a source for CAR-NK cells further supports standardized manufacturing and consistent product quality
- Overall, the movement toward universal CAR-NK platforms is reshaping cell therapy development and accelerating clinical adoption
CAR-NK Cell Therapy Market Dynamics
Driver
Rising Prevalence of Cancer and Limitations of Existing Immunotherapies
- The increasing global burden of cancer, particularly hematological malignancies and resistant solid tumors, is a key driver for the growth of the CAR-NK Cell Therapy market. Limitations associated with conventional chemotherapy, radiotherapy, and even CAR-T therapies have intensified the demand for safer and more effective immunotherapies
- For instance, in January 2024, the U.S. National Cancer Institute highlighted CAR-NK therapies as a promising next-generation immunotherapy due to their reduced toxicity and broader applicability compared to CAR-T cells, supporting increased research funding and clinical trials
- CAR-NK therapies offer innate tumor-killing capabilities without prior antigen sensitization, improving therapeutic versatility
- Lower incidence of severe immune-related adverse events enhances patient safety and treatment continuity
- Growing investment from pharmaceutical and biotechnology companies accelerates clinical development pipelines
- Increasing regulatory support for advanced cell therapies further strengthens market growth prospects
Restraint/Challenge
Manufacturing Complexity, Limited Persistence, and High Development Costs
- Despite strong clinical potential, the CAR-NK Cell Therapy market faces challenges related to complex manufacturing processes, limited in-vivo persistence of NK cells, and high research and development costs. These factors can slow large-scale commercialization and accessibility
- For instance, clinical studies published in 2022 indicated that CAR-NK cells exhibit shorter persistence in patients compared to CAR-T cells, necessitating repeated dosing or combination approaches, which increases overall treatment costs
- Manufacturing CAR-NK cells requires specialized infrastructure, skilled personnel, and stringent quality controls
- Shorter cell persistence can impact long-term efficacy, particularly in solid tumor indications
- High production costs may limit adoption in cost-sensitive healthcare systems
- Addressing these challenges through improved cell engineering, cytokine support strategies, and cost-efficient manufacturing platforms will be critical for sustained market expansion
CAR-NK Cell Therapy Market Scope
The market is segmented on the basis of indication and end user.
- By Indication
On the basis of indication, the CAR-NK Cell Therapy market is segmented into hematological malignancies, solid tumors, and others. The hematological malignancies segment dominated the largest market revenue share of 58.4% in 2025, driven by the high clinical success of CAR-NK therapies in blood cancers such as leukemia, lymphoma, and multiple myeloma. CAR-NK cells demonstrate strong cytotoxic activity against CD19 and CD22 antigens, which are commonly expressed in hematological cancers. The lower incidence of cytokine release syndrome and graft-versus-host disease compared to CAR-T therapies has accelerated adoption in this segment. Favorable clinical trial outcomes, faster regulatory pathways, and higher response rates further support dominance. Increasing prevalence of blood cancers globally and strong investment from biotechnology companies strengthen market leadership. Established clinical protocols and higher physician confidence also contribute to sustained revenue generation.
The solid tumors segment is expected to witness the fastest CAGR of 29.6% from 2026 to 2033, driven by ongoing innovation aimed at overcoming tumor microenvironment barriers. Advances in genetic engineering, multi-target CAR constructs, and cytokine-enhanced NK cells are improving efficacy in solid tumors such as glioblastoma, ovarian cancer, and pancreatic cancer. Rising unmet medical need and limited effectiveness of existing immunotherapies accelerate research focus. Increasing clinical trials, strategic collaborations, and growing funding for solid tumor immuno-oncology support rapid expansion. Improved infiltration and persistence strategies are further enhancing therapeutic outcomes. This segment represents a major future growth opportunity for the CAR-NK Cell Therapy market.
- By End User
On the basis of end user, the CAR-NK Cell Therapy market is segmented into hospitals, specialized cancer centers, research & academic institutes, and others. The specialized cancer centers segment dominated the largest market revenue share of 46.9% in 2025, due to their advanced infrastructure, expertise in cellular immunotherapy, and ability to manage complex oncology treatments. These centers are primary sites for CAR-NK clinical trials and early commercial adoption. Availability of specialized oncologists, cell processing units, and multidisciplinary care teams supports high treatment volumes. Strong participation in translational research and partnerships with biotech firms further reinforce dominance. Patient preference for specialized oncology care and access to innovative therapies also drive revenue concentration.
The hospitals segment is projected to register the fastest CAGR of 24.8% from 2026 to 2033, driven by expanding adoption of advanced cell therapies beyond tertiary cancer centers. Increasing hospital investments in oncology infrastructure and cell therapy units are improving accessibility. Growing collaborations with academic institutions and biotech companies enable hospitals to offer CAR-NK treatments. Rising patient inflow, improved reimbursement frameworks, and increasing physician awareness contribute to rapid growth. Hospitals are increasingly positioning themselves as comprehensive oncology hubs. This shift significantly accelerates market expansion across broader healthcare settings.
CAR-NK Cell Therapy Market Regional Analysis
- North America dominated the CAR-NK Cell Therapy market with the largest revenue share of approximately 38.6% in 2025
- Supported by strong R&D infrastructure, high oncology research funding, early adoption of advanced cell therapies, and the robust presence of biotechnology and pharmaceutical companies
- The region benefits from well-established clinical trial networks, favorable regulatory pathways, and significant investments in immuno-oncology research, which continue to accelerate the development and commercialization of CAR-NK therapies
U.S. CAR-NK Cell Therapy Market Insight
The U.S. CAR-NK Cell Therapy market accounted for the majority of revenue within North America in 2025, driven by extensive research activities, rising prevalence of hematological malignancies, and strong funding from both public and private sectors. Leading academic research institutes, biotech firms, and pharmaceutical companies in the U.S. are actively engaged in developing next-generation CAR-NK therapies with improved safety and efficacy profiles. Additionally, the presence of advanced manufacturing capabilities and a growing number of clinical trials is further strengthening the country’s leadership in this market.
Europe CAR-NK Cell Therapy Market Insight
The Europe CAR-NK Cell Therapy market is expected to expand at a notable CAGR during the forecast period, driven by increasing investments in cell and gene therapy research, supportive regulatory initiatives, and growing collaboration between academic institutions and biotechnology companies. Several European countries are actively funding oncology research programs, which is fostering innovation in CAR-NK cell therapies. The rising incidence of cancer and the demand for safer alternatives to CAR-T therapies are further contributing to market growth across the region.
U.K. CAR-NK Cell Therapy Market Insight
The U.K. CAR-NK Cell Therapy market is anticipated to witness steady growth over the forecast period, supported by strong government backing for advanced therapies, a well-developed life sciences ecosystem, and increasing participation in clinical trials. The country’s focus on translational research and precision medicine is encouraging the development of novel CAR-NK platforms, while partnerships between universities, research centers, and biotech firms continue to enhance innovation and commercialization prospects.
Germany CAR-NK Cell Therapy Market Insight
The Germany CAR-NK Cell Therapy market is projected to grow at a considerable CAGR, driven by its strong biomedical research infrastructure, advanced healthcare system, and increasing emphasis on innovative cancer treatments. Germany’s leadership in clinical research, combined with rising investments in cell therapy manufacturing and quality control, is supporting the adoption and development of CAR-NK therapies, particularly in specialized oncology centers.
Asia-Pacific CAR-NK Cell Therapy Market Insight
The Asia-Pacific CAR-NK Cell Therapy market is expected to be the fastest-growing region during the forecast period, registering a high CAGR. This growth is driven by expanding biotechnology sectors, increasing government support for cell therapy research, rising cancer prevalence, and improving clinical trial capabilities in countries such as China, Japan, and South Korea. The region is also witnessing growing investments in local manufacturing and R&D, which is enhancing accessibility to advanced cell therapies.
Japan CAR-NK Cell Therapy Market Insight
The Japan CAR-NK Cell Therapy market is gaining momentum due to strong government initiatives supporting regenerative medicine, a well-established regulatory framework for advanced therapies, and increasing research into next-generation immunotherapies. Japan’s aging population and rising cancer burden are further driving demand for innovative and safer cell-based treatments, positioning CAR-NK therapy as a promising option in both clinical research and future clinical applications.
China CAR-NK Cell Therapy Market Insight
The China CAR-NK Cell Therapy market accounted for a significant share of Asia-Pacific revenue in 2025, supported by rapid expansion of the biotechnology industry, increasing government funding for oncology research, and a growing number of clinical trials. China’s focus on developing domestic cell therapy capabilities, along with strong collaboration between research institutes and biotech companies, is accelerating the advancement and potential commercialization of CAR-NK therapies across the country.
CAR-NK Cell Therapy Market Share
The CAR-NK Cell Therapy industry is primarily led by well-established companies, including:
• Nkarta Therapeutics (U.S.)
• Takeda Pharmaceutical (Japan)
• Cellular Biomedicine Group (U.S.)
• Celyad Oncology (Belgium)
• Gilead Sciences (U.S.)
• Bellicum Pharmaceuticals (U.S.)
• Kiadis Pharma (Netherlands)
• Tessa Therapeutics (Singapore)
• Allogene Therapeutics (U.S.)
• ImmunityBio (U.S.)
• Karus Therapeutics (U.S.)
• Mabwell Biotech (China)
• Innovative Cellular Therapeutics (China)
• Precision BioSciences (U.S.)
• Sorrento Therapeutics (U.S.)
• Adaptimmune Therapeutics (U.K.)
• Sangamo Therapeutics (U.S.)
• Cellular Biologics (China)
• Freenome Therapeutics (U.S.)
Latest Developments in Global CAR-NK Cell Therapy Market
- In November 2024, Fate Therapeutics, a clinical-stage biopharmaceutical company, launched FT522, an off-the-shelf CD19-targeted CAR-NK cell therapy incorporating alloimmune defense receptor technology, designed to persist and selectively deplete pathogenic B cells in patients with relapsed or refractory B-cell lymphoma and autoimmune diseases, demonstrating promising safety and efficacy in early clinical evaluation. This launch reflects the broader trend toward “off-the-shelf” allogeneic CAR-NK therapies that aim to reduce treatment complexity compared with individualized cell therapies
- In February 2025, global CAR-NK development was highlighted as part of broader NK cell therapy market growth, with FT522 being evaluated in Phase I trials showing rapid and sustained B-cell depletion, alongside plans to expand clinical development into additional indications including autoimmune disorders. These interim results underscore CAR-NK’s potential to transform standards of care in multiple disease areas
SKU-
Accédez en ligne au rapport sur le premier cloud mondial de veille économique
- Tableau de bord d'analyse de données interactif
- Tableau de bord d'analyse d'entreprise pour les opportunités à fort potentiel de croissance
- Accès d'analyste de recherche pour la personnalisation et les requêtes
- Analyse de la concurrence avec tableau de bord interactif
- Dernières actualités, mises à jour et analyse des tendances
- Exploitez la puissance de l'analyse comparative pour un suivi complet de la concurrence
Méthodologie de recherche
La collecte de données et l'analyse de l'année de base sont effectuées à l'aide de modules de collecte de données avec des échantillons de grande taille. L'étape consiste à obtenir des informations sur le marché ou des données connexes via diverses sources et stratégies. Elle comprend l'examen et la planification à l'avance de toutes les données acquises dans le passé. Elle englobe également l'examen des incohérences d'informations observées dans différentes sources d'informations. Les données de marché sont analysées et estimées à l'aide de modèles statistiques et cohérents de marché. De plus, l'analyse des parts de marché et l'analyse des tendances clés sont les principaux facteurs de succès du rapport de marché. Pour en savoir plus, veuillez demander un appel d'analyste ou déposer votre demande.
La méthodologie de recherche clé utilisée par l'équipe de recherche DBMR est la triangulation des données qui implique l'exploration de données, l'analyse de l'impact des variables de données sur le marché et la validation primaire (expert du secteur). Les modèles de données incluent la grille de positionnement des fournisseurs, l'analyse de la chronologie du marché, l'aperçu et le guide du marché, la grille de positionnement des entreprises, l'analyse des brevets, l'analyse des prix, l'analyse des parts de marché des entreprises, les normes de mesure, l'analyse globale par rapport à l'analyse régionale et des parts des fournisseurs. Pour en savoir plus sur la méthodologie de recherche, envoyez une demande pour parler à nos experts du secteur.
Personnalisation disponible
Data Bridge Market Research est un leader de la recherche formative avancée. Nous sommes fiers de fournir à nos clients existants et nouveaux des données et des analyses qui correspondent à leurs objectifs. Le rapport peut être personnalisé pour inclure une analyse des tendances des prix des marques cibles, une compréhension du marché pour d'autres pays (demandez la liste des pays), des données sur les résultats des essais cliniques, une revue de la littérature, une analyse du marché des produits remis à neuf et de la base de produits. L'analyse du marché des concurrents cibles peut être analysée à partir d'une analyse basée sur la technologie jusqu'à des stratégies de portefeuille de marché. Nous pouvons ajouter autant de concurrents que vous le souhaitez, dans le format et le style de données que vous recherchez. Notre équipe d'analystes peut également vous fournir des données sous forme de fichiers Excel bruts, de tableaux croisés dynamiques (Fact book) ou peut vous aider à créer des présentations à partir des ensembles de données disponibles dans le rapport.