Global Digeorge Syndrome Drug Market
Taille du marché en milliards USD
TCAC :
% 
 USD
365.40 Million
USD
608.70 Million
2024
2032
USD
365.40 Million
USD
608.70 Million
2024
2032
| 2025 –2032 | |
| USD 365.40 Million | |
| USD 608.70 Million | |
|
|
|
|
Global DiGeorge Syndrome Drug Market, By Drug Class (Immunosuppressants, Hormone Replacements, Calcium Supplements, Others), Route of Administration (Oral, Intravenous, Others), End-Users (Clinics, Hospitals, Others), Distribution Channel (Hospital Pharmacy, Retail Pharmacy, Online Pharmacy) - Industry Trends and Forecast to 2032
DiGeorge Syndrome Drug Market Size
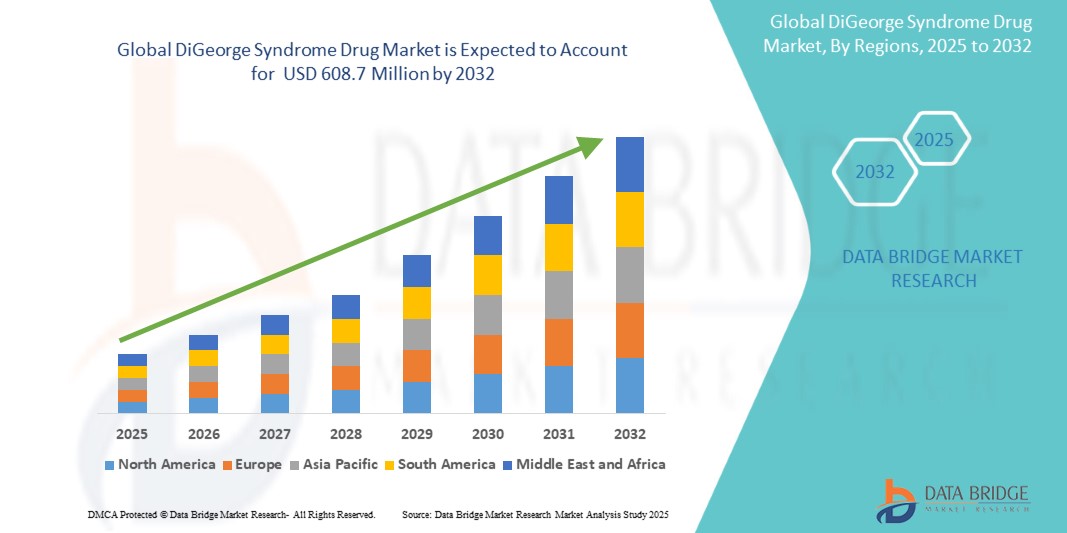
- The global DiGeorge Syndrome Drug Market was valued at USD 365.4 million in 2024 and is expected to reach USD 608.7 million by 2032.
- During the forecast period of 2025 to 2032, the market is projected to grow at a CAGR of 6.5%, primarily fueled by improved diagnostics and increasing awareness of rare genetic disorders.
- This growth is driven by factors such as rising cases of DiGeorge syndrome, advancements in genetic screening, and the development of targeted and supportive drug therapies.
DiGeorge Syndrome Drug Market Analysis
- The global DiGeorge Syndrome Drug Market is expected to register significant growth in the forecast period of 2025 to 2032. Data Bridge Market Research analyzes the market to grow at a CAGR of 6.5% during this period.
- DiGeorge syndrome, also known as 22q11.2 deletion syndrome, is a rare genetic disorder that can cause heart defects, poor immune function, cleft palate, and low levels of calcium in the blood. Early diagnosis and comprehensive treatment are critical to managing symptoms and improving quality of life.
- Factors such as the increasing prevalence of congenital disorders, expanding research on rare diseases, supportive healthcare policies, and growing investments in orphan drug development are accelerating market growth globally
Report Scope and Global DiGeorge Syndrome Drug Market Segmentation
|
Attributes |
DiGeorge Syndrome Drug Market Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include import export analysis, production capacity overview, production consumption analysis, price trend analysis, climate change scenario, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Global DiGeorge Syndrome Drug Market Trends
“Increased Emphasis on Genetic Screening and Immunological Management”
- A significant trend in the DiGeorge syndrome drug market is the growing focus on early genetic testing and targeted immunological therapies to address the multi-systemic manifestations of the disorder.
- Treatment regimens are evolving to include personalized care plans, including thymus transplantation, calcium supplementation, antipsychotic therapies, and prophylactic antibiotics for immune-compromised patients.
- For example, improved clinical guidelines now recommend chromosomal microarray testing and FISH (fluorescent in situ hybridization) as standard diagnostic tools for early detection of 22q11.2 deletion syndrome.
- Furthermore, multidisciplinary care involving cardiology, endocrinology, psychiatry, and immunology is being emphasized to optimize outcomes and manage long-term complications.
- The rising incidence of congenital heart defects, developmental delays, and immune deficiencies linked with DiGeorge syndrome is creating heightened awareness and demand for appropriate drug therapies.
Global DiGeorge Syndrome Drug Market Dynamics
Driver
“Growing Diagnosis Rates and Clinical Awareness of 22q11.2 Deletion Syndrome”
DiGeorge syndrome, or 22q11.2 deletion syndrome, is a rare genetic disorder with a broad spectrum of clinical manifestations, including congenital heart defects, immunodeficiency, hypocalcemia, and neurodevelopmental disorders.
For instance:
- According to a 2023 article in Genetics in Medicine, DiGeorge syndrome occurs in approximately 1 in 3,000 to 6,000 live births, but actual incidence may be higher due to underdiagnosis in less developed regions.
- Common symptoms such as recurrent infections, speech and learning delays, and palatal abnormalities are increasingly being recognized, leading to earlier intervention and improved quality of life.
“Increased Government Funding and Support Programs for Rare Diseases”
Government and non-profit initiatives are expanding support for early diagnosis, drug development, and care infrastructure targeting rare diseases like DiGeorge syndrome.
For instance:
In 2022, various national rare disease frameworks were launched in the U.S., Europe, and Asia-Pacific to fund gene therapy trials and promote the availability of orphan drugs for conditions like 22q11.2 deletion syndrome.
Opportunity
“Advances in Gene Therapy and Immunomodulatory Treatments”
The DiGeorge syndrome drug market is gaining momentum due to breakthroughs in gene editing, thymus regeneration, and immune system modulation therapies.
For instance:
- Ongoing clinical trials are exploring the safety and efficacy of exogenous thymus tissue implantation and CRISPR-based gene correction techniques.
- Additionally, targeted immunoglobulin therapies and prophylactic antibiotics are being evaluated to reduce infection risks in patients with severe immunodeficiency.
- As understanding of the genetic and immunologic basis of the disease deepens, opportunities for personalized therapeutic strategies continue to expand.
Restraint/Challenge
“High Cost of Specialized Treatments and Limited Access in Developing Regions”
- The availability of advanced treatments like gene therapy, thymus transplantation, and long-term immunological care is often limited by high costs and lack of infrastructure in many parts of the world.
- Moreover, delayed diagnosis and lack of specialized care centers in resource-constrained settings further challenge disease management.
For example:
- Despite the promise of precision medicine approaches, access to next-generation diagnostics and therapies may remain out of reach for many patients due to financial and logistical barriers, especially in low- and middle-income countries
Global DiGeorge Syndrome Drug Market Scope
The market is segmented on the basis of drug class, route of administration, end-users, and distribution channel.
|
Segmentation |
Sub-Segmentation |
|
• By Drug
|
|
|
Route of Administration |
|
|
End-Users |
|
|
Distribution Channel |
|
Global DiGeorge Syndrome Drug Market Regional Analysis
“North America is the Dominant Region in the Global DiGeorge Syndrome Drug Market”
- North America leads the global DiGeorge Syndrome Drug market due to its sophisticated healthcare infrastructure, high disease awareness, and strong focus on rare genetic disorders.
- The United States holds the largest market share, driven by advanced genomic research, established newborn screening programs, and comprehensive multidisciplinary treatment frameworks for congenital conditions.
- The presence of key pharmaceutical and biotech companies focused on rare disease drug development supports market growth.
- Favorable government incentives, orphan drug designations, and regulatory support by the FDA contribute to the faster approval and adoption of targeted therapies for DiGeorge syndrome.
“Asia-Pacific is Projected to Register the Highest Growth Rate”
- The Asia-Pacific region is anticipated to witness the fastest growth in the DiGeorge Syndrome Drug market owing to rising awareness of genetic disorders, expanding pediatric care infrastructure, and increased access to diagnostic testing.
- Countries like China, India, Japan, and South Korea are spearheading growth through increased public health spending and collaboration with international research initiatives.
- Government-backed early genetic screening programs and better access to immunological and endocrinological care are improving patient outcomes.
- Japan, with its robust rare disease management programs and focus on precision medicine, is an early adopter of newer treatment protocols.
- Growing partnerships between global biopharma companies and regional institutions are further enhancing access to cutting-edge therapies.
DiGeorge Syndrome Drug Market Share
The competitive landscape of the market provides in-depth insights into major players, including their company profiles, financial overviews, R&D investment, product pipeline, global footprint, production capabilities, strategic collaborations, competitive advantages, and challenges—especially related to therapies addressing DiGeorge syndrome’s immunological, cardiac, and endocrine manifestations.
The Major Market Leaders Operating in the Market Include:
- Pfizer Inc.
- Johnson & Johnson Services, Inc.
- Novartis AG
- F. Hoffmann-La Roche Ltd.
- Bristol-Myers Squibb Company
- Takeda Pharmaceutical Company Limited
- Amgen Inc.
- BioMarin Pharmaceutical Inc.
- Ipsen Biopharmaceuticals, Inc.
- Teva Pharmaceutical Industries Ltd.
- Sanofi S.A.
- Vertex Pharmaceuticals Incorporated
- CSL Behring
- Regeneron Pharmaceuticals, Inc.
- Grifols, S.A.
- Zydus Lifesciences
- Aurobindo Pharma
- Dr. Reddy’s Laboratories Ltd.
- Glenmark Pharmaceuticals
- Lupin Pharmaceuticals, Inc.
Latest Developments in Global DiGeorge Syndrome Drug Market
- In June 2023, a collaborative research study published in Orphanet Journal of Rare Diseases highlighted key challenges in managing DiGeorge syndrome, including fragmented care coordination, delayed diagnosis, and limited access to multidisciplinary support teams.
- It emphasized the need for integrated clinical pathways, broader awareness among pediatricians, and enhanced funding for gene-based and immunomodulatory therapies to improve early treatment outcomes and long-term quality of life.
SKU-
Accédez en ligne au rapport sur le premier cloud mondial de veille économique
- Tableau de bord d'analyse de données interactif
- Tableau de bord d'analyse d'entreprise pour les opportunités à fort potentiel de croissance
- Accès d'analyste de recherche pour la personnalisation et les requêtes
- Analyse de la concurrence avec tableau de bord interactif
- Dernières actualités, mises à jour et analyse des tendances
- Exploitez la puissance de l'analyse comparative pour un suivi complet de la concurrence
Méthodologie de recherche
La collecte de données et l'analyse de l'année de base sont effectuées à l'aide de modules de collecte de données avec des échantillons de grande taille. L'étape consiste à obtenir des informations sur le marché ou des données connexes via diverses sources et stratégies. Elle comprend l'examen et la planification à l'avance de toutes les données acquises dans le passé. Elle englobe également l'examen des incohérences d'informations observées dans différentes sources d'informations. Les données de marché sont analysées et estimées à l'aide de modèles statistiques et cohérents de marché. De plus, l'analyse des parts de marché et l'analyse des tendances clés sont les principaux facteurs de succès du rapport de marché. Pour en savoir plus, veuillez demander un appel d'analyste ou déposer votre demande.
La méthodologie de recherche clé utilisée par l'équipe de recherche DBMR est la triangulation des données qui implique l'exploration de données, l'analyse de l'impact des variables de données sur le marché et la validation primaire (expert du secteur). Les modèles de données incluent la grille de positionnement des fournisseurs, l'analyse de la chronologie du marché, l'aperçu et le guide du marché, la grille de positionnement des entreprises, l'analyse des brevets, l'analyse des prix, l'analyse des parts de marché des entreprises, les normes de mesure, l'analyse globale par rapport à l'analyse régionale et des parts des fournisseurs. Pour en savoir plus sur la méthodologie de recherche, envoyez une demande pour parler à nos experts du secteur.
Personnalisation disponible
Data Bridge Market Research est un leader de la recherche formative avancée. Nous sommes fiers de fournir à nos clients existants et nouveaux des données et des analyses qui correspondent à leurs objectifs. Le rapport peut être personnalisé pour inclure une analyse des tendances des prix des marques cibles, une compréhension du marché pour d'autres pays (demandez la liste des pays), des données sur les résultats des essais cliniques, une revue de la littérature, une analyse du marché des produits remis à neuf et de la base de produits. L'analyse du marché des concurrents cibles peut être analysée à partir d'une analyse basée sur la technologie jusqu'à des stratégies de portefeuille de marché. Nous pouvons ajouter autant de concurrents que vous le souhaitez, dans le format et le style de données que vous recherchez. Notre équipe d'analystes peut également vous fournir des données sous forme de fichiers Excel bruts, de tableaux croisés dynamiques (Fact book) ou peut vous aider à créer des présentations à partir des ensembles de données disponibles dans le rapport.