Global Poc Immunoassay Analyzer Expansion Market
Taille du marché en milliards USD
TCAC :
% 
 USD
607.66 Million
USD
1,420.68 Million
2025
2033
USD
607.66 Million
USD
1,420.68 Million
2025
2033
| 2026 –2033 | |
| USD 607.66 Million | |
| USD 1,420.68 Million | |
|
|
|
|
Global POC Immunoassay Analyzer Expansion Market Segmentation, By Product Type (Portable Analyzers, Benchtop Analyzers, and Multiplex Analyzers), Application ( Cardiac Markers, Infectious Disease Testing, Oncology, Endocrinology, and Other Diagnostic Tests), End-User ( Hospitals, Diagnostic Laboratories, Ambulatory Care Centers, and Research Institutes) - Industry Trends and Forecast to 2033
POC Immunoassay Analyzer Expansion Market Size
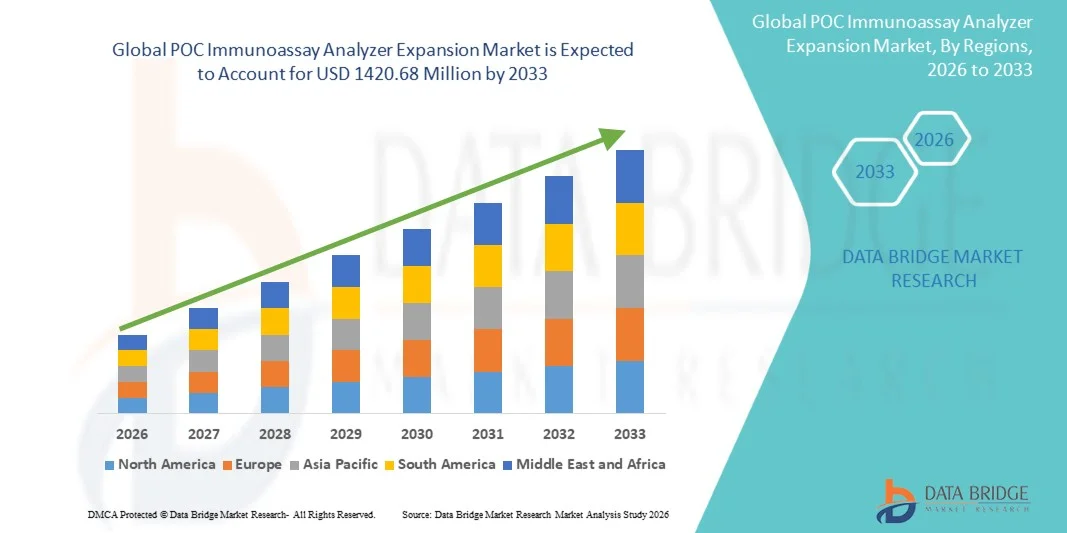
- The global POC immunoassay analyzer expansion market size was valued at USD 607.66 Million in 2025 and is expected to reach USD 1420.68 Million by 2033, at a CAGR of 11.20% during the forecast period
- The market growth is largely driven by advancements in point-of-care diagnostic technologies, increasing demand for rapid and accurate testing, and growing adoption of decentralized healthcare models, enabling faster clinical decision-making in hospitals, clinics, and diagnostic centers
- Furthermore, rising prevalence of chronic and infectious diseases, increasing focus on early disease detection, and the need for cost-effective and portable diagnostic solutions are accelerating the uptake of POC Immunoassay Analyzer Expansion solutions, thereby significantly boosting the overall growth of the POC Immunoassay Analyzer Expansion Market
POC Immunoassay Analyzer Expansion Market Analysis
- POC Immunoassay Analyzers, which enable rapid, accurate, and on-site detection of biomarkers for infectious and chronic diseases, are increasingly essential in modern healthcare systems due to the rising need for point-of-care testing, faster diagnosis, and improved patient outcomes
- The market growth is primarily driven by technological advancements in portable immunoassay analyzers, increasing prevalence of chronic and infectious diseases, rising demand for decentralized diagnostics, and growing focus on early disease detection, which are accelerating the adoption of POC Immunoassay Analyzer Expansion solutions
- North America dominated the POC Immunoassay Analyzer Expansion market with a revenue share of approximately 37.6% in 2025, supported by advanced healthcare infrastructure, high adoption of point-of-care diagnostics, and strong presence of leading market players in the U.S.
- Asia-Pacific is expected to be the fastest-growing region during the forecast period, owing to expanding healthcare infrastructure, increasing government initiatives for early disease detection, rising disposable incomes, and growing awareness of point-of-care diagnostic solutions in countries such as China and India
- The Benchtop Analyzers segment dominated the largest market revenue share of 44.5% in 2025, driven by their high throughput, ability to handle multiple tests simultaneously, and integration with hospital laboratory information systems
Report Scope and POC Immunoassay Analyzer Expansion Market Segmentation
|
Attributes |
POC Immunoassay Analyzer Expansion Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
POC Immunoassay Analyzer Expansion Market Trends
“Technological Advancements and Growing Adoption in POC Immunoassay Analyzer Expansion”
- A significant and accelerating trend in the global POC Immunoassay Analyzer Expansion market is the development of portable, rapid, and user-friendly analyzers that enable faster diagnosis and testing at the point of care
- For instance, in February 2025, Abbott Laboratories launched its next-generation i-STAT Alinity Analyzer in Southeast Asia, enabling hospitals and clinics to perform multiple immunoassays with faster turnaround times and minimal sample volume
- The integration of multi-parameter testing capabilities and enhanced connectivity features is allowing healthcare providers to monitor patient data in real time and make timely clinical decisions
- Rising demand for decentralized testing, especially in remote and under-served areas, is driving innovations in compact and easy-to-use POC immunoassay devices
- The trend toward efficient, rapid, and accessible testing solutions is reshaping diagnostic workflows, enabling hospitals, clinics, and emergency care centers to improve patient outcomes while reducing operational delays
POC Immunoassay Analyzer Expansion Market Dynamics
Driver
“Increasing Need for Rapid Diagnosis and Efficient Healthcare Delivery”
- The growing prevalence of chronic diseases, infectious illnesses, and acute medical conditions is driving demand for POC immunoassay analyzers that can deliver fast, accurate results at the bedside or in outpatient settings
- For instance, in July 2024, Siemens Healthineers deployed its Atellica VTLi POC immunoassay systems across multiple clinics in India, reducing diagnostic turnaround times by over 30% and improving patient management efficiency
- The rise of home healthcare and outpatient diagnostic services is further encouraging adoption of compact and easy-to-use analyzers
- Government initiatives to improve healthcare accessibility in emerging markets are also propelling growth, as rapid POC testing reduces the burden on centralized laboratories
- Advancements in test sensitivity, multiplexing capabilities, and connectivity with hospital information systems are additional factors driving market expansion
Restraint/Challenge
“High Costs, Regulatory Compliance, and Accuracy Concerns”
- The high cost of advanced POC immunoassay analyzers and associated reagents can be a barrier to adoption, particularly for small clinics or healthcare facilities in developing regions
- For instance, in March 2025, a chain of diagnostic centers in Indonesia delayed deployment of new POC analyzers due to the high initial investment and the need to comply with national regulatory approvals for clinical use
- Stringent regulatory requirements and the need for device validation in multiple regions can delay market entry and increase operational costs
- Potential inaccuracies or variability in test results compared to centralized laboratory analyzers may create hesitation among healthcare providers
- Training personnel to operate the devices effectively and ensuring quality control are additional challenges, particularly in under-resourced healthcare settings
- Overcoming these barriers through cost-effective device options, regulatory harmonization, and comprehensive training programs is essential for sustained growth in the POC Immunoassay Analyzer Expansion market
POC Immunoassay Analyzer Expansion Market Scope
The market is segmented on the basis of product type, application, and end-user.
• By Product Type
On the basis of product type, the POC Immunoassay Analyzer Expansion market is segmented into Portable Analyzers, Benchtop Analyzers, and Multiplex Analyzers. The Benchtop Analyzers segment dominated the largest market revenue share of 44.5% in 2025, driven by their high throughput, ability to handle multiple tests simultaneously, and integration with hospital laboratory information systems. Benchtop analyzers are widely adopted in hospitals and large diagnostic laboratories due to their reliability, scalability, and compatibility with a broad range of immunoassays. The ease of calibration, precise measurements, and reduced turnaround time for critical tests such as cardiac markers and infectious disease diagnostics further fuels demand. Established providers offer customizable platforms with advanced features, including automated sample handling and result reporting. The segment benefits from strong institutional purchasing, long product lifecycles, and continuous software updates. In addition, ongoing training and technical support for laboratory staff enhance operational efficiency and adoption rates. With growing demand for rapid and accurate immunoassays in emerging markets, benchtop analyzers remain a preferred choice in clinical settings, consolidating their market leadership.
The Multiplex Analyzers segment is expected to witness the fastest CAGR of 22.3% from 2026 to 2033, driven by their capability to detect multiple biomarkers from a single sample simultaneously, offering significant efficiency for oncology, infectious diseases, and endocrine testing. These analyzers save time, reduce sample volume requirements, and improve diagnostic accuracy, making them increasingly popular in research institutes and advanced hospital laboratories. Rising prevalence of chronic diseases and the need for rapid, high-throughput diagnostics further accelerate growth. Technological advancements, such as automated microfluidic systems and integrated data analytics, are enhancing their performance and usability. Increasing adoption in emerging markets due to cost-efficiency and the ability to run diverse test panels further supports expansion. Strategic partnerships and investments by key players to enhance multiplex capabilities also contribute to the segment’s strong growth trajectory.
• By Application
On the basis of application, the POC Immunoassay Analyzer Expansion market is segmented into Cardiac Markers, Infectious Disease Testing, Oncology, Endocrinology, and Other Diagnostic Tests. The Infectious Disease Testing segment dominated the market in 2025 with a revenue share of 41.8%, driven by heightened testing requirements during the COVID-19 pandemic and subsequent awareness of rapid diagnostics for diseases like influenza, HIV, and hepatitis. Hospitals and diagnostic laboratories prioritize rapid, sensitive, and specific assays for early detection, which has increased adoption of POC immunoassay analyzers. Integration with cloud-based reporting systems and mobile platforms has improved efficiency and reduced turnaround times. Continuous introduction of new kits, automation, and multiplexing capabilities further strengthen the segment. Government initiatives and reimbursement policies in developed regions also support widespread deployment. The segment benefits from robust clinical validation, regulatory approvals, and strong presence of key market players offering comprehensive solutions.
The Oncology segment is expected to witness the fastest CAGR of 23.1% from 2026 to 2033, fueled by rising cancer incidence worldwide and the growing need for early detection and personalized treatment strategies. Multiplex immunoassay analyzers are increasingly used for biomarker panels to identify cancer subtypes, monitor treatment response, and guide therapy selection. Innovations such as high-sensitivity assays, automated sample processing, and integration with electronic medical records enhance diagnostic utility. Increasing investment in oncology research, partnerships between diagnostic and pharmaceutical companies, and expansion of testing capabilities in emerging markets further drive growth.
• By End-User
On the basis of end-user, the POC Immunoassay Analyzer Expansion market is segmented into Hospitals, Diagnostic Laboratories, Ambulatory Care Centers, and Research Institutes. The Hospitals segment held the largest market share of 46.3% in 2025, driven by large-scale adoption of POC analyzers for rapid diagnostics in emergency, ICU, and outpatient departments. Hospitals prefer benchtop and multiplex analyzers for their robustness, reliability, and ability to handle high sample volumes. Investments in hospital laboratories, regulatory compliance, and integration with laboratory information systems have further strengthened demand.
The Research Institutes segment is expected to witness the fastest CAGR of 21.8% from 2026 to 2033, due to increased use of immunoassay analyzers in clinical research, biomarker discovery, and high-throughput drug development. Rising funding in biotechnology and life sciences research, along with adoption of portable and multiplex analyzers, supports rapid growth in this segment. Moreover, the growing emphasis on personalized medicine and advanced diagnostic techniques is driving demand for highly sensitive and efficient analyzers. In addition, collaborations between research institutes and diagnostic solution providers are facilitating faster deployment and utilization of these analyzers.
POC Immunoassay Analyzer Expansion Market Regional Analysis
- North America dominated the POC Immunoassay Analyzer Expansion market with a revenue share of approximately 37.6% in 2025, supported by advanced healthcare infrastructure, high adoption of point-of-care diagnostics, and strong presence of leading market players in the U.S.
- Hospitals, clinics, and diagnostic centers in the region highly value the rapid testing capabilities, accuracy, and ease of use offered by POC immunoassay analyzers, enabling timely disease detection and improved patient outcomes
- This widespread adoption is further supported by continuous investments in healthcare infrastructure, increasing demand for early diagnosis, and the growing preference for decentralized diagnostic solutions, establishing POC immunoassay analyzers as critical tools in clinical and research settings
U.S. POC Immunoassay Analyzer Expansion Market Insight
The U.S. POC Immunoassay Analyzer Expansion market captured the largest revenue share within North America in 2025, driven by high adoption of point-of-care diagnostic solutions in hospitals, clinics, and research laboratories. Increasing government initiatives for early disease detection, along with ongoing innovations by leading diagnostic equipment manufacturers, is propelling market growth. The focus on rapid and accurate testing to improve patient management further contributes to market expansion.
Europe POC Immunoassay Analyzer Expansion Market Insight
The Europe POC Immunoassay Analyzer Expansion market is projected to expand at a substantial CAGR during the forecast period, primarily driven by rising awareness of early disease detection, increasing adoption of point-of-care diagnostics, and supportive healthcare policies. Countries across the region are witnessing growing investments in hospital infrastructure and laboratory modernization, boosting demand for POC immunoassay analyzers in clinical settings.
U.K. POC Immunoassay Analyzer Expansion Market Insight
The U.K. POC Immunoassay Analyzer Expansion market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by increasing adoption of rapid diagnostic solutions in hospitals and diagnostic centers. Growing awareness of preventive healthcare, alongside government support for point-of-care testing programs, is expected to further stimulate market growth.
Germany POC Immunoassay Analyzer Expansion Market Insight
The Germany POC Immunoassay Analyzer Expansion market is expected to expand at a considerable CAGR during the forecast period, fueled by a strong healthcare infrastructure, increasing use of rapid diagnostic technologies, and investments in clinical laboratory modernization. Rising demand for accurate and rapid diagnostic solutions in hospitals and research institutes is driving market growth.
Asia-Pacific POC Immunoassay Analyzer Expansion Market Insight
The Asia-Pacific POC Immunoassay Analyzer Expansion market is expected to be the fastest-growing region during the forecast period, owing to expanding healthcare infrastructure, increasing government initiatives for early disease detection, rising disposable incomes, and growing awareness of point-of-care diagnostic solutions in countries such as China and India. Growing investments in hospitals and diagnostic laboratories, along with increasing adoption of advanced POC diagnostic technologies, are accelerating market growth across the region.
China POC Immunoassay Analyzer Expansion Market Insight
The China POC Immunoassay Analyzer Expansion market accounted for the largest revenue share in Asia-Pacific in 2025, attributed to rapid expansion of healthcare facilities, increasing adoption of point-of-care diagnostics, and strong government support for early disease detection programs. Hospitals and diagnostic centers are increasingly deploying POC immunoassay analyzers to improve patient management, optimize clinical workflows, and enhance testing accuracy.
India POC Immunoassay Analyzer Expansion Market Insight
The India POC Immunoassay Analyzer Expansion market is projected to witness significant growth during the forecast period, driven by rising healthcare expenditure, expanding hospital and diagnostic networks, growing awareness about early disease diagnosis, and increasing adoption of point-of-care diagnostic technologies. Improvements in healthcare infrastructure and government initiatives promoting early testing are further supporting market growth.
POC Immunoassay Analyzer Expansion Market Share
The POC Immunoassay Analyzer Expansion industry is primarily led by well-established companies, including:
- Abbott (U.S.)
- Roche Diagnostics (Switzerland)
- Siemens Healthineers (Germany)
- Quidel Corporation (U.S.)
- bioMérieux (France)
- Ortho Clinical Diagnostics (U.S.)
- DiaSorin S.p.A. (Italy)
- Thermo Fisher Scientific (U.S.)
- Becton Dickinson (U.S.)
- Sysmex Corporation (Japan)
- Trinity Biotech (Ireland)
- EKF Diagnostics (U.K.)
- Nova Biomedical (U.S.)
- LumiraDx (U.K.)
- Sekisui Diagnostics (Japan)
- Hologic, Inc. (U.S.)
- Beckman Coulter (U.S.)
- F. Hoffmann-La Roche AG (Switzerland)
- Werfen Life Sciences (Spain)
Latest Developments in Global POC Immunoassay Analyzer Expansion Market
- In December 2023, Siemens Healthineers expanded its point‑of‑care immunoassay portfolio with a new test for cardiac biomarkers designed to deliver improved turnaround times for acute cardiac events and broaden support for rapid diagnosis in decentralized healthcare settings
- In January 2024, Quidel Corporation received FDA clearance for an integrated point‑of‑care molecular instrument capable of detecting a range of infectious pathogens with high sensitivity and speed, enhancing the company’s diagnostic offerings at the point of care
- In February 2024, Abbott announced the launch of a new rapid molecular POC test for detection of respiratory syncytial virus (RSV) and influenza A/B, aimed at delivering faster diagnostic results in clinical settings, particularly relevant to POC immunoassay testing environments
- In July 2024, Siemens Healthineers highlighted advanced automation and workflow improvements across its diagnostic portfolio at the AACC/ADLM 2024 conference, including capabilities that enhance POC immunoassay performance and integration into broader clinical workflows
SKU-
Accédez en ligne au rapport sur le premier cloud mondial de veille économique
- Tableau de bord d'analyse de données interactif
- Tableau de bord d'analyse d'entreprise pour les opportunités à fort potentiel de croissance
- Accès d'analyste de recherche pour la personnalisation et les requêtes
- Analyse de la concurrence avec tableau de bord interactif
- Dernières actualités, mises à jour et analyse des tendances
- Exploitez la puissance de l'analyse comparative pour un suivi complet de la concurrence
Méthodologie de recherche
La collecte de données et l'analyse de l'année de base sont effectuées à l'aide de modules de collecte de données avec des échantillons de grande taille. L'étape consiste à obtenir des informations sur le marché ou des données connexes via diverses sources et stratégies. Elle comprend l'examen et la planification à l'avance de toutes les données acquises dans le passé. Elle englobe également l'examen des incohérences d'informations observées dans différentes sources d'informations. Les données de marché sont analysées et estimées à l'aide de modèles statistiques et cohérents de marché. De plus, l'analyse des parts de marché et l'analyse des tendances clés sont les principaux facteurs de succès du rapport de marché. Pour en savoir plus, veuillez demander un appel d'analyste ou déposer votre demande.
La méthodologie de recherche clé utilisée par l'équipe de recherche DBMR est la triangulation des données qui implique l'exploration de données, l'analyse de l'impact des variables de données sur le marché et la validation primaire (expert du secteur). Les modèles de données incluent la grille de positionnement des fournisseurs, l'analyse de la chronologie du marché, l'aperçu et le guide du marché, la grille de positionnement des entreprises, l'analyse des brevets, l'analyse des prix, l'analyse des parts de marché des entreprises, les normes de mesure, l'analyse globale par rapport à l'analyse régionale et des parts des fournisseurs. Pour en savoir plus sur la méthodologie de recherche, envoyez une demande pour parler à nos experts du secteur.
Personnalisation disponible
Data Bridge Market Research est un leader de la recherche formative avancée. Nous sommes fiers de fournir à nos clients existants et nouveaux des données et des analyses qui correspondent à leurs objectifs. Le rapport peut être personnalisé pour inclure une analyse des tendances des prix des marques cibles, une compréhension du marché pour d'autres pays (demandez la liste des pays), des données sur les résultats des essais cliniques, une revue de la littérature, une analyse du marché des produits remis à neuf et de la base de produits. L'analyse du marché des concurrents cibles peut être analysée à partir d'une analyse basée sur la technologie jusqu'à des stratégies de portefeuille de marché. Nous pouvons ajouter autant de concurrents que vous le souhaitez, dans le format et le style de données que vous recherchez. Notre équipe d'analystes peut également vous fournir des données sous forme de fichiers Excel bruts, de tableaux croisés dynamiques (Fact book) ou peut vous aider à créer des présentations à partir des ensembles de données disponibles dans le rapport.