Gsa And Benelux Point Care Testing Poct Market
Taille du marché en milliards USD
TCAC :
% 
 USD
3.63 Billion
USD
9.07 Billion
2024
2032
USD
3.63 Billion
USD
9.07 Billion
2024
2032
| 2025 –2032 | |
| USD 3.63 Billion | |
| USD 9.07 Billion | |
|
|
|
|
Marché des tests au point de service (POCT) de la GSA et du Benelux, par type de produit (produits de surveillance du glucose, produits de dépistage des maladies infectieuses, produits de surveillance cardiométabolique, produits de test de grossesse et de fertilité, produits de test d'hématologie, produits de surveillance de la coagulation, produits de test de drogues d'abus (DOA), produits de test d'analyse d'urine, produits de test de cholestérol, produits de test de marqueurs tumoraux/cancer, produits de test d'occultation fécale, et autres), par plateforme (tests de flux latéral/tests d'immunochromatographie, immuno-essais, bandelettes réactives, diagnostics moléculaires, tests de chimie clinique, microfluidique, hématologie, autres), par application (glycémie, maladies infectieuses, surveillance des signes vitaux, surveillance cardiaque, coagulation, hématologie, surveillance non invasive de la SpO2, transfusion sanguine, PCO2 non invasive) Surveillance, analyse du sang total, autres), par mode de prescription (tests sur ordonnance et tests en vente libre), par utilisateur final (hôpitaux, soins à domicile, cliniques, laboratoires, centres de diagnostic, laboratoires de pathologie, centres de chirurgie ambulatoire, centres de soins pour personnes âgées, autres), par canal de distribution (appel d'offres direct, vente au détail, vente en ligne, etc.) - Tendances et prévisions du secteur jusqu'en 2032
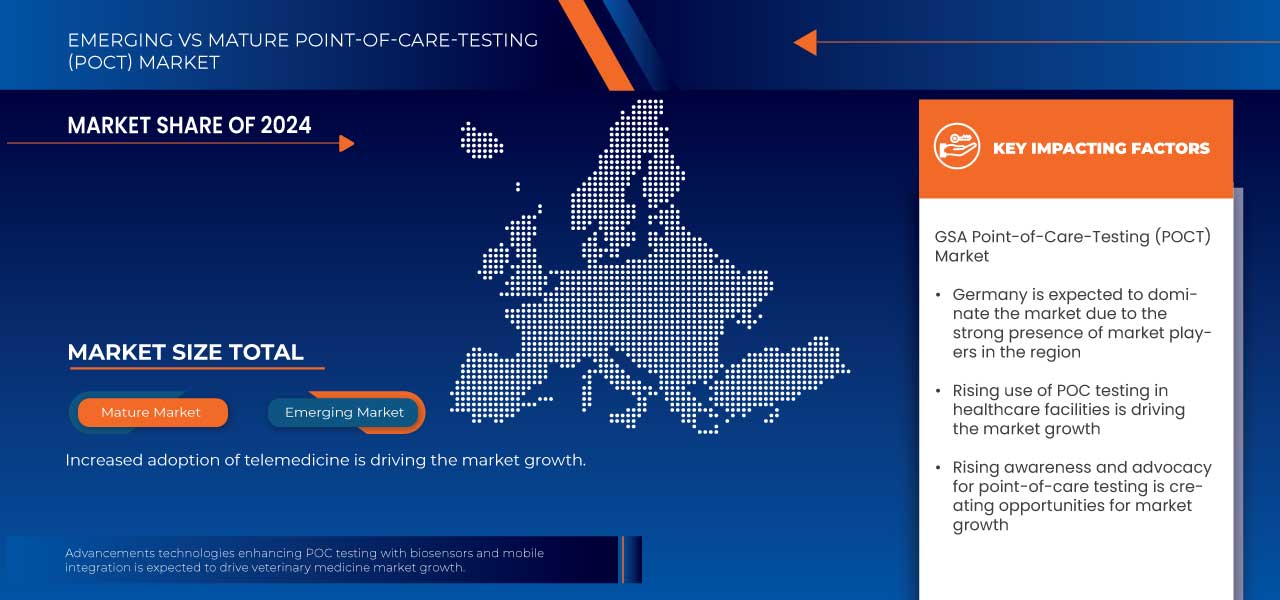
Taille du marché des tests au point de service (POCT)
- La taille du marché des tests au point de service (POCT) de la GSA était évaluée à 3,63 milliards USD en 2024 et devrait atteindre 9,07 milliards USD d'ici 2032 , à un TCAC de 12,1 % au cours de la période de prévision.
- La taille du marché des tests au point de service (POCT) au Benelux était évaluée à 882 millions USD en 2024 et devrait atteindre 1 819 millions USD d'ici 2032 , à un TCAC de 9,5 % au cours de la période de prévision.
- Cette croissance est due à des facteurs tels que la demande croissante de résultats de diagnostic rapides, la prévalence croissante des maladies chroniques et infectieuses et les progrès des technologies de diagnostic portables.
Analyse du marché des tests au point de service (POCT)
- Les dispositifs de diagnostic au point d'intervention (POCT) jouent un rôle essentiel dans l'obtention rapide de résultats diagnostiques sur le lieu de soins ou à proximité, améliorant ainsi considérablement la prise de décision clinique dans divers contextes de soins. Ces tests sont largement utilisés dans la prise en charge des maladies chroniques, des maladies infectieuses et des urgences.
- La demande de solutions POCT dans les régions GSA et Benelux est principalement motivée par la charge croissante des maladies chroniques, la croissance de la population âgée et une forte poussée en faveur de modèles de soins de santé décentralisés et centrés sur le patient.
- L'Allemagne devrait dominer le marché POCT avec une part de 28,04 % , dans la région en raison de son infrastructure de soins de santé bien établie, de son débit de diagnostic élevé et de l'adoption de technologies médicales avancées.
- Les pays du Benelux devraient connaître une forte croissance du marché, tirée par des initiatives gouvernementales favorables, une augmentation des dépenses de santé et une adoption croissante du POCT dans les environnements cliniques et de soins à domicile.
- Le segment des tests de glycémie, avec une part de 34,08 %, devrait dominer le marché POCT dans la région, en raison de la forte prévalence du diabète et de la demande d'outils de surveillance en temps réel qui permettent aux patients de gérer leur maladie.
Portée du rapport et segmentation du marché des tests au point de service (POCT)
|
Attributs |
Aperçu du marché des microscopes ophtalmiques opérationnels |
|
Segments couverts |
|
|
Pays couverts |
GSA
BENELUX
|
|
Principaux acteurs du marché |
|
|
Opportunités de marché |
|
|
Ensembles d'informations de données à valeur ajoutée |
Outre les informations sur les scénarios de marché tels que la valeur marchande, le taux de croissance, la segmentation, la couverture géographique et les principaux acteurs, les rapports de marché organisés par Data Bridge Market Research comprennent également une analyse des importations et des exportations, un aperçu de la capacité de production, une analyse de la consommation de production, une analyse des tendances des prix, un scénario de changement climatique, une analyse de la chaîne d'approvisionnement, une analyse de la chaîne de valeur, un aperçu des matières premières/consommables, des critères de sélection des fournisseurs, une analyse PESTLE, une analyse Porter et un cadre réglementaire. |
Tendances du marché des tests au point de service (POCT)
« Progrès en matière de diagnostic rapide et de tests décentralisés en Afrique du Sud et au Benelux »
- L’une des tendances majeures qui façonnent le marché POCT dans les régions GSA et Benelux est l’intégration croissante de la connectivité numérique, des capteurs miniaturisés et des capacités de test multiplex dans les appareils de point de service.
- Ces innovations améliorent la vitesse et la précision du diagnostic, permettant aux prestataires de soins de santé de prendre des décisions opportunes, en particulier dans les situations d'urgence, la gestion des maladies chroniques et le dépistage des maladies infectieuses.
- Par exemple, les systèmes POCT équipés d'une transmission de données sans fil et d'une intégration de dossiers médicaux électroniques (DME) sont de plus en plus utilisés dans les établissements de soins primaires en Allemagne et aux Pays-Bas pour rationaliser la surveillance des patients et réduire les visites à l'hôpital.
- Ces avancées révolutionnent les diagnostics décentralisés, soutiennent les modèles de soins basés sur la valeur et accélèrent la transition vers des soins de santé centrés sur le patient dans les régions GSA et Benelux.
- L'importance croissante accordée aux soins de santé préventifs, combinée au fort soutien gouvernemental à la détection précoce des maladies, continue d'alimenter la demande de solutions POCT de nouvelle génération avec des performances analytiques et une convivialité améliorées.
Dynamique du marché des tests au point de service (POCT)
Conducteur
« Utilisation croissante des tests POC dans les établissements de santé »
- L'utilisation croissante des tests au point de service (POC) dans les établissements de santé révolutionne la façon dont les prestataires de soins de santé diagnostiquent et gèrent les maladies.
- Face au besoin croissant de résultats diagnostiques rapides et précis, les professionnels de santé se tournent vers les tests sur le lieu de soins pour améliorer la prise en charge des patients. Cette évolution est particulièrement marquée aux urgences, en consultation externe et dans les centres de diagnostic, où la rapidité de prise de décision est cruciale.
- Les tests POC permettent aux prestataires de soins de santé d'obtenir des résultats immédiats au point de service, éliminant ainsi le besoin pour les patients d'attendre les résultats de laboratoire et réduisant considérablement le temps de diagnostic et de traitement.
- De plus, les tests POC sont hautement portables et conviviaux, ce qui les rend idéaux pour une utilisation dans divers contextes de soins de santé, des cliniques rurales aux grands hôpitaux
- La prévalence croissante des maladies chroniques et la demande croissante de solutions de soins de santé efficaces alimentent davantage l'adoption des tests POC, car ils permettent une meilleure surveillance des maladies, en particulier dans des conditions comme le diabète, les maladies cardiovasculaires et les maladies infectieuses.
- Alors que les établissements de santé intègrent de plus en plus ces technologies dans leurs flux de travail, la demande de tests au point de service continue de croître, agissant comme un moteur clé de l'expansion du marché.
Par exemple,
- En mars 2024, selon le NCBI, la rapidité d'exécution des examens POCT améliore la prise de décision clinique, réduit le temps de gestion et optimise l'efficacité du triage, notamment aux urgences. Sa flexibilité s'étend aux milieux préhospitaliers, aux hôpitaux de campagne, ainsi qu'en cas de catastrophe, de rassemblement de masse ou dans les zones surpeuplées.
- En novembre 2024, selon un article publié par OBJECTIVE Investment Banking & Valuation, l'adoption des tests au point d'intervention (POCT) a révolutionné les soins de santé en permettant d'obtenir des diagnostics directement sur le lieu de prise en charge du patient. Leur rapidité, leur praticité et leur efficacité en font un outil précieux pour les urgences, les consultations externes et les interventions à distance. La demande de diagnostics rapides augmente à mesure que la demande augmente.
- L'adoption croissante des tests au point de service (POC) dans les établissements de santé transforme le diagnostic et la prise en charge des maladies. Soucieux d'obtenir des résultats rapides et précis, les professionnels de santé utilisent les tests au point de service pour améliorer la prise en charge des patients.
Opportunité
« Sensibilisation et plaidoyer accrus en faveur des tests au point de service »
- À mesure que les professionnels de la santé, les décideurs politiques et le grand public sont mieux informés des avantages du POCT – tels que des résultats rapides, une meilleure gestion des patients, des délais d’exécution réduits et le potentiel de tests décentralisés – la demande pour ces solutions augmente.
- Les efforts de plaidoyer des groupes de patients, des organisations professionnelles et des organismes industriels soulignent davantage la valeur du POCT dans divers contextes cliniques, notamment les services d'urgence, les soins primaires et même dans les milieux communautaires.
- Cette compréhension et ce soutien accrus se traduisent par une plus grande volonté d'investir et d'intégrer le POCT dans les flux de travail de santé existants, élargissant ainsi la taille et la portée du marché en favorisant une adoption et une utilisation accrues.
Par exemple,
- En avril 2023, le NCBI a indiqué qu'une écrasante majorité de médecins généralistes allemands (93 %) considéraient les tests de dépistage au point d'intervention (TPPI) comme des outils diagnostiques précieux dans leur pratique. Ils ont principalement cité la possibilité de prendre des décisions immédiates pour la prise en charge des patients et l'amélioration de la fiabilité diagnostique comme les principaux avantages de l'utilisation des TPI.
- Selon un article intitulé « Tests diagnostiques au point de service en soins primaires aux Pays-Bas » publié par Open Repository, les professionnels de santé ont de plus en plus recours aux tests diagnostiques au point de service (POC) pour prendre des décisions thérapeutiques. Le principal avantage de ces tests réside dans leur capacité à fournir des résultats rapides, directement sur place ou à proximité du patient.
- De plus, cette sensibilisation et ce plaidoyer accrus peuvent conduire à des changements politiques favorables et à des initiatives de financement favorisant une mise en œuvre plus large du dépistage sur le lieu de soins. Les gouvernements et les systèmes de santé de l'Afrique du Sud et du Benelux, conscients du potentiel du dépistage sur le lieu de soins pour améliorer l'efficacité et l'accès aux diagnostics, pourraient allouer davantage de ressources à l'adoption de ces technologies.
- Le plaidoyer peut également inciter les organismes de réglementation à simplifier les processus d'approbation des dispositifs POCT innovants, accélérant ainsi leur mise sur le marché. Cela crée un environnement plus propice à la croissance du marché, encourageant les acteurs établis comme les nouveaux entrants à investir dans le développement et le déploiement de solutions POCT avancées pour répondre à la demande croissante.
Retenue/Défi
« Préoccupations liées à la sécurité et à la confidentialité des données »
- À mesure que de plus en plus d'appareils POCT s'intègrent aux plateformes numériques, la collecte, la transmission et le stockage d'informations sensibles sur les patients augmentent les risques d'accès non autorisé et de violation de données. Les données de santé, qui incluent les données médicales personnelles, nécessitent une protection stricte en vertu de lois telles que la loi HIPAA et le RGPD, ce qui engendre des difficultés de conformité supplémentaires pour les fabricants et les prestataires de soins.
- L’absence de cadres de sécurité robustes et de protocoles de cryptage dans certains appareils POCT amplifie encore ces préoccupations.
- De plus, l'hésitation des patients à partager leurs données médicales en raison des risques pour la vie privée peut freiner leur adoption généralisée, notamment dans les régions où la confiance dans les solutions de santé numérique est limitée. Le développement continu de systèmes sécurisés et respectueux de la vie privée est essentiel, mais les préoccupations actuelles freinent l'innovation et l'adoption sur le marché des POCT en Afrique du Sud et au Benelux.
Par exemple,
- En juillet 2021, selon le blog publié par MDPI, un aspect pertinent à prendre en compte concernant les risques liés à la cybersécurité et à la confidentialité concerne les systèmes de soins de santé au point de service (POC), largement utilisés dans les hôpitaux. Ces systèmes sont des plateformes intégrant des appareils et des applications pour collecter, traiter et visualiser des données. L'utilisation de grandes quantités de données, contenant des informations de santé personnelles et des données médicales sensibles, transmises via divers systèmes POC, plateformes d'analyse back-end, postes de travail utilisateurs et smartphones, met en évidence l'existence de multiples menaces pouvant entraîner des fuites de données ou des incidents de violation mettant en danger la vie des patients. Les tests au point de service sont vulnérables aux fuites de données, ce qui freine la croissance du marché.
- Les préoccupations en matière de sécurité et de confidentialité des données constituent des défis majeurs pour le marché des tests au point de service (POCT) de la GSA et du Benelux. À mesure que de plus en plus d'appareils se connectent aux plateformes numériques, le risque d'accès non autorisé et de violation de données augmente, notamment en ce qui concerne les informations sensibles des patients. La conformité aux réglementations telles que HIPAA et RGPD complexifie les choses pour les fabricants et les prestataires de soins. L'absence de sécurité renforcée de certains appareils et les préoccupations des patients en matière de confidentialité peuvent freiner l'adoption, notamment dans les régions où la confiance dans les solutions de santé numérique est limitée. Le développement de systèmes sécurisés et respectueux de la confidentialité est essentiel pour surmonter ces obstacles.
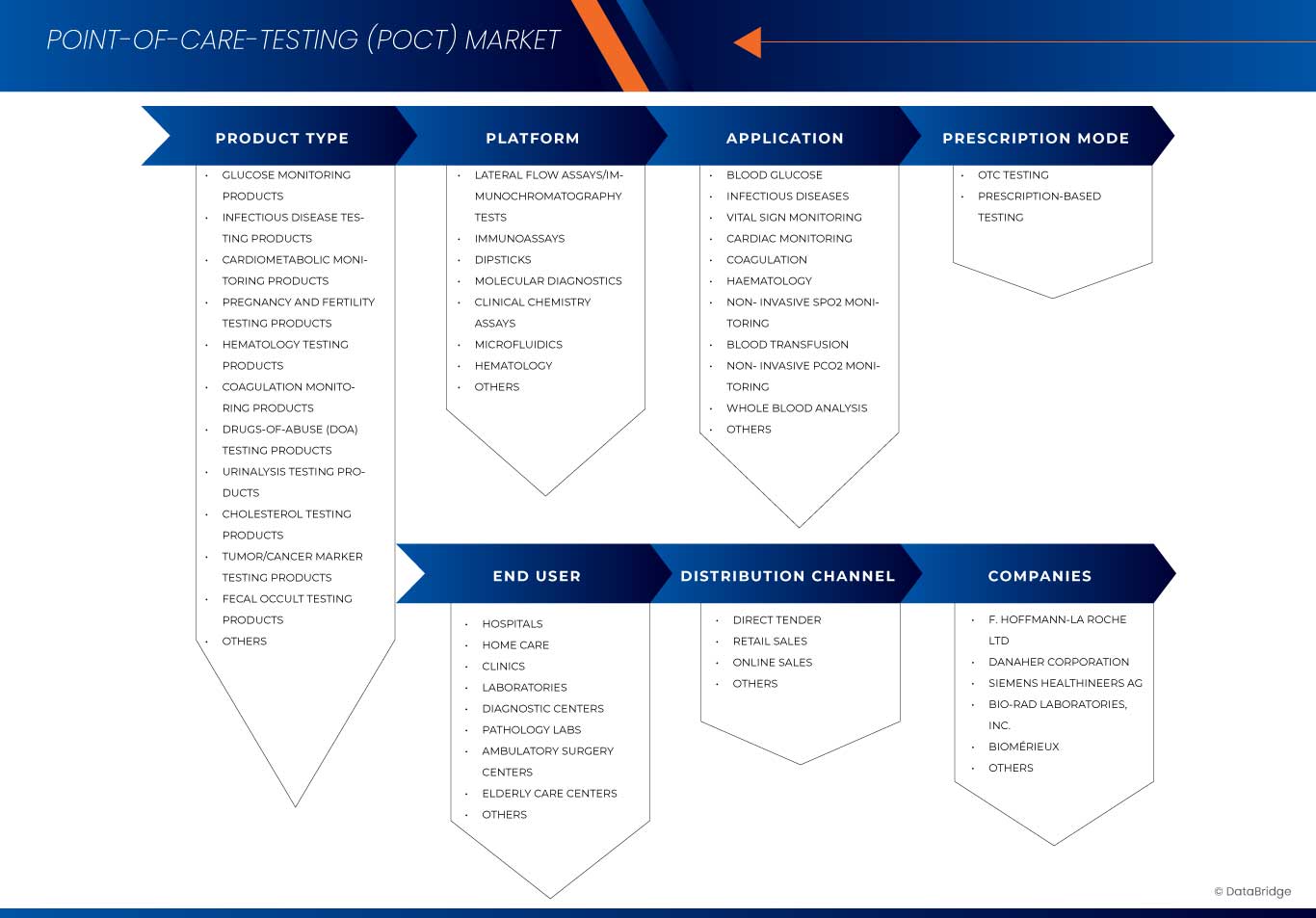
Portée du marché des tests au point de service (POCT)
Le marché est segmenté en fonction de l'application, du type de produit, de la technologie, du type de grossissement, de l'utilisateur final et du canal de distribution.
|
Segmentation |
Sous-segmentation |
|
Par type de produit |
|
|
Par plateforme |
|
|
Par application |
|
|
Par mode Prescription
|
|
|
Par utilisateur final |
|
|
Par canal de distribution
|
|
En 2025, les produits de surveillance de la glycémie devraient dominer le marché avec une part de marché plus importante dans le segment des types de produits.
Le segment des produits de surveillance de la glycémie devrait dominer le marché des tests au point de service (poct) avec la plus grande part de 34,08 % en 2025 en raison de la forte prévalence du diabète et du besoin fréquent de tests de glycémie pratiques et immédiats.
Les tests d'immunochromatographie/d'analyse de flux latéral devraient représenter la plus grande part au cours de la période de prévision dans le segment des plateformes.
En 2025, le segment des tests d'immunochromatographie/dosages à flux latéral devrait dominer le marché avec la plus grande part de marché de 37,28 % en raison de leur simplicité, de leurs résultats rapides, de leur rentabilité et de leur adéquation à une large gamme d'applications de diagnostic en dehors des laboratoires traditionnels.
Analyse régionale du marché des tests au point de service (POCT)
« L'Allemagne détient la plus grande part du marché des tests au point de service (POCT) »
- L'Allemagne domine le marché des tests au point de service (POCT) avec une part de 82,06 % , dans la région GSA, grâce à une infrastructure de soins de santé très développée, à l'adoption généralisée de technologies de diagnostic innovantes et à un fort accent mis sur la médecine préventive et personnalisée.
- Le pays détient une part importante en raison de la demande croissante de solutions de diagnostic rapides et précises, en particulier dans la gestion des maladies chroniques comme le diabète, les maladies cardiovasculaires et les infections respiratoires.
- Des cadres de remboursement favorables, des initiatives de santé numérique soutenues par le gouvernement et des investissements importants dans la R&D en matière de soins de santé renforcent encore la croissance du marché.
- En outre, la mise en œuvre croissante du POCT dans les services d'urgence, les établissements de soins primaires et les soins à domicile, associée à l'accent mis sur la réduction de la charge hospitalière, alimente l'expansion du marché dans toute l'Allemagne.
« Les Pays-Bas devraient enregistrer le TCAC le plus élevé sur le marché des tests au point de service (POCT) »
- Les Pays-Bas devraient connaître une forte croissance du marché des tests au point de service (POCT) avec une part de 54,40 % , grâce à leur forte concentration sur les soins de santé numériques, la détection précoce des maladies et les modèles de soins centrés sur le patient.
- Le vieillissement de la population du pays et la prévalence croissante de maladies chroniques telles que le diabète, les maladies cardiovasculaires et les troubles respiratoires alimentent la demande de solutions de diagnostic rapides et décentralisées.
- Avec un système de soins primaires bien établi et des politiques de santé progressistes, les Pays-Bas restent un pays clé pour l'adoption des technologies POCT, en particulier dans les établissements de santé communautaires, les cliniques de médecine générale (MG) et les services de soins à domicile.
- L'intégration croissante des dispositifs POCT aux dossiers médicaux électroniques (DME), le soutien des assureurs maladie à l'innovation diagnostique et la participation active aux initiatives européennes en matière de technologies de la santé renforcent encore les perspectives du marché.
- Les collaborations entre les institutions universitaires, les startups technologiques et les sociétés de diagnostic accélèrent l'innovation et le développement local de solutions POCT avancées et conviviales adaptées au système de santé néerlandais.
Part de marché des tests au point de service (POCT)
Le paysage concurrentiel du marché fournit des détails par concurrent. Il comprend la présentation de l'entreprise, ses données financières, son chiffre d'affaires, son potentiel de marché, ses investissements en recherche et développement, ses nouvelles initiatives commerciales, sa présence mondiale, ses sites et installations de production, ses capacités de production, ses forces et faiblesses, le lancement de nouveaux produits, leur ampleur et leur portée, ainsi que la domination de ses applications. Les données ci-dessus ne concernent que les activités des entreprises par rapport à leur marché.
Les principaux leaders du marché opérant sur le marché sont :
- F. Hoffmann-La Roche SA (Suisse)
- Danaher Corporation (États-Unis)
- Siemens Healthineers AG (Allemagne)
- Bio-Rad Laboratories, Inc. (États-Unis)
- BIOMÉRIEUX (France)
- BD (États-Unis)
- QuidelOrtho Corporation (États-Unis)
- Hologic, Inc. (États-Unis)
- Thermo Fisher Scientific Inc. (États-Unis)
- Abbott (États-Unis)
- Werfen (Espagne)
- QIAGEN (Allemagne)
- Sysmex Corporation (Japon)
- EKF Diagnostics Holdings plc. (Royaume-Uni)
- Sekisui Diagnostics (États-Unis)
- Meridian Bioscience (États-Unis)
- binx health, inc. (États-Unis)
- Trinity Biotech (Irlande)
- Sienco, Inc. (États-Unis)
- ACCUBIOTECH CO., LTD. (Chine)
Dernières évolutions sur le marché des tests au point de service (POCT) en Afrique du Sud et au Benelux
- En décembre 2023, Roche a acquis certains composants de la technologie innovante Point of Care de LumiraDx, enrichissant ainsi son portefeuille de diagnostics. Cette acquisition permet des tests plus rapides et plus abordables dans de nombreux domaines thérapeutiques. L'intégration de la plateforme LumiraDx améliorera l'accès des patients à des résultats diagnostiques rapides et précis dans les établissements de santé décentralisés du monde entier.
- En janvier 2025, Danaher Corporation a conclu un partenariat d'investissement avec Innovaccer Inc., une entreprise spécialisée dans l'IA médicale. Cette collaboration vise à accélérer l'adoption de diagnostics de précision et de soins à valeur ajoutée en fournissant aux professionnels de santé des données patients unifiées et des analyses avancées, améliorant ainsi les résultats des patients grâce à des interventions personnalisées et rapides.
- En octobre 2024, bioMérieux a appelé à une collaboration multisectorielle pour faire progresser l'agenda mondial sur la résistance aux antimicrobiens (RAM). L'entreprise souligne l'urgence d'une collaboration entre les professionnels de santé, les gouvernements et l'industrie pour lutter contre la RAM, en promouvant des solutions diagnostiques innovantes et un usage responsable des antimicrobiens afin de lutter contre cette menace sanitaire mondiale croissante.
- En mars 2024, Bio-Rad a lancé la solution de sérotypage des salmonelles XP-Design Assay pour des tests de sécurité alimentaire rapides et précis. Cette solution offre une approche flexible pour identifier les sérotypes de salmonelles après un dépistage initial. Prête à l'emploi, elle contient des oligonucléotides (amorces et sondes) spécifiques au sérotype cible, garantissant une spécificité élevée et réduisant le risque de réactions croisées, pour des résultats fiables.
SKU-
Accédez en ligne au rapport sur le premier cloud mondial de veille économique
- Tableau de bord d'analyse de données interactif
- Tableau de bord d'analyse d'entreprise pour les opportunités à fort potentiel de croissance
- Accès d'analyste de recherche pour la personnalisation et les requêtes
- Analyse de la concurrence avec tableau de bord interactif
- Dernières actualités, mises à jour et analyse des tendances
- Exploitez la puissance de l'analyse comparative pour un suivi complet de la concurrence
Table des matières
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF THE GSA AND BENELUX POINT-OF-CARE-TESTING (POCT) MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 YEARS CONSIDERED FOR THE STUDY
2.3 CURRENCY AND PRICING
2.4 DBMR TRIPOD DATA VALIDATION MODEL
2.5 MULTIVARIATE MODELLING
2.6 PRODUCT TYPE LIFELINE CURVE
2.7 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.8 DBMR MARKET POSITION GRID
2.9 MARKET END USER COVERAGE GRID
2.1 SECONDARY SOURCES
2.11 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTAL ANALYSIS
4.2 PORTERS FIVE FORCES ANALYSIS
4.3 MICRO AND MACRO ECONOMIC FACTORS
4.4 PENETRATION AND GROWTH PROSPECT MAPPING
4.5 KEY PRICING STRATEGIES
4.6 COST ANALYSIS BREAKDOWN
4.7 OPPORTUNITY MAP ANALYSIS
4.8 VALUE CHAIN ANALYSIS
4.9 HEALTHCARE ECONOMY
4.1 TARIFFS
4.10.1 DEFINITION AND IMPORTANCE OF TARIFFS IN THE HEALTHCARE SECTOR
4.10.2 GLOBAL VS. REGIONAL TARIFF STRUCTURES
4.10.3 IMPACT OF TARIFFS ON HEALTHCARE COSTS AND ACCESSIBILITY
4.10.4 TARIFF REGULATIONS IN KEY MARKETS
4.10.4.1 MEDICARE/MEDICAID TARIFF POLICIES
4.10.4.2 CMS PRICING MODELS
4.10.4.3 OTHERS
4.10.5 TARIFFS ON MEDICAL DEVICES & EQUIPMENT
4.10.5.1 IMPORT/EXPORT DUTIES ON MEDICAL EQUIPMENT
4.10.5.2 IMPACT ON PRICING AND AVAILABILITY OF HIGH-END MEDICAL TECHNOLOGY
4.10.5.3 CASE STUDIES OF TARIFF CHANGES AFFECTING THE INDUSTRY
4.10.6 COST BURDEN ON HOSPITALS AND HEALTHCARE FACILITIES
4.10.7 TARIFF EXEMPTIONS AND INCENTIVES
4.10.8 DUTY-FREE IMPORTS FOR ESSENTIAL MEDICINES AND VACCINES
4.10.9 16.9 IMPACT OF TRADE WARS ON THE HEALTHCARE SUPPLY CHAIN
4.10.10 ROLE OF FREE TRADE AGREEMENTS (FTAS) IN REDUCING TARIFFS
5 CUSTOMIZATION
5.1 LEADING DIGITAL HEALTH PLATFORMS IN EACH COUNTRY –
5.2 POCT DATA INTEGRATION USE CASES –
5.3 INFORMATICS PLATFORMS & TELEMEDICINE INTEGRATION:
6 REGULATORY
6.1 REGULATORY GSA
6.2 REGULATORY BENELUX
7 MARKET OVERVIEW
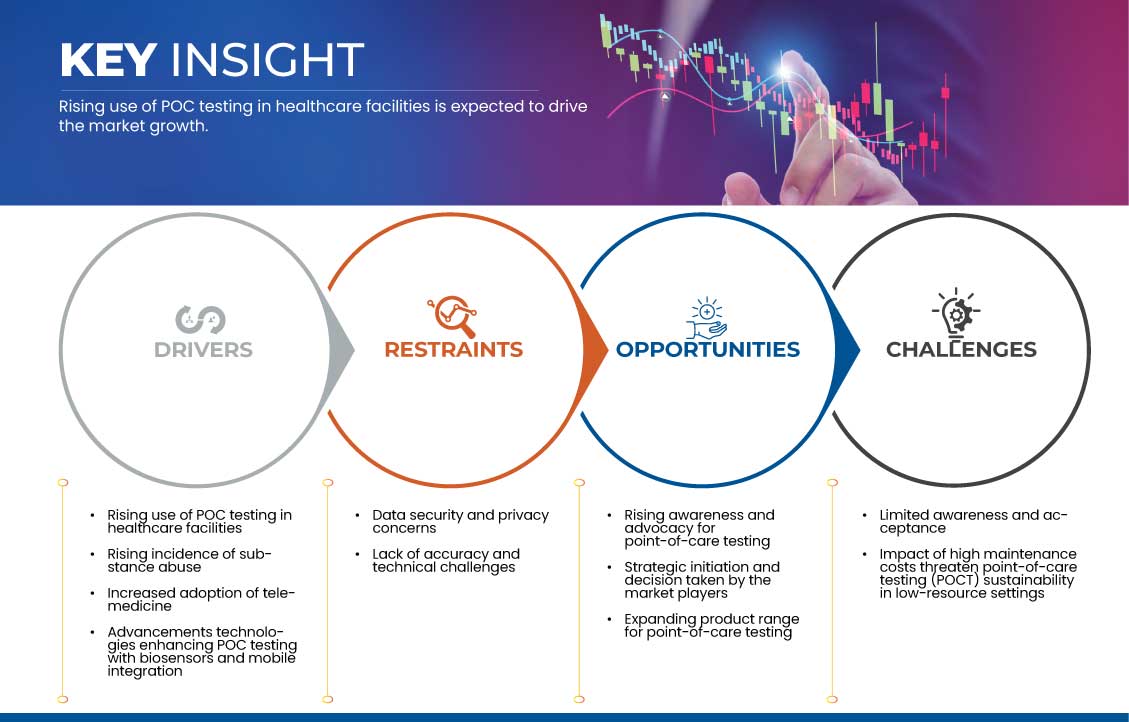
7.1 DRIVERS
7.1.1 RISING USE OF POC TESTING IN HEALTHCARE FACILITIES
7.1.2 RISING INCIDENCE OF SUBSTANCE ABUSE
7.1.3 INCREASED ADOPTION OF TELEMEDICINE
7.1.4 ADVANCEMENTS TECHNOLOGIES ENHANCING POC TESTING WITH BIOSENSORS AND MOBILE INTEGRATION
7.2 RESTRAINTS
7.2.1 DATA SECURITY AND PRIVACY CONCERNS
7.2.2 ACCURACY LIMITATIONS AND TECHNICAL BARRIERS
7.3 OPPORTUNITIES
7.3.1 RISING AWARENESS AND ADVOCACY FOR POINT-OF-CARE TESTING
7.3.2 STRATEGIC INITIATION AND DECISION TAKEN BY THE MARKET PLAYERS
7.3.3 EXPANDING PRODUCT RANGE FOR POINT-OF-CARE TESTING
7.4 CHALLENGES
7.4.1 LIMITED AWARENESS AND ACCEPTANCE OF POINT-OF-CARE (POC) TESTING
7.4.2 IMPACT OF HIGH MAINTENANCE COSTS THREATEN POINT-OF-CARE TESTING (POCT) SUSTAINABILITY IN LOW-RESOURCE SETTINGS
8 GSA POINT-OF-CARE TESTING (POCT) MARKET, BY PRODUCT TYPE
8.1 OVERVIEW
8.2 GLUCOSE MONITORING PRODUCTS
8.2.1 STRIPS
8.2.2 METERS
8.2.3 LANCETS AND LANCING DEVICES
8.3 INFECTIOUS DISEASE TESTING PRODUCTS
8.3.1 COVID-19
8.3.2 HIV TESTING PRODUCTS
8.3.2.1 TESTING REAGENTS
8.3.2.2 TESTING EQUIPMENT
8.3.3 RESPIRATORY INFECTION TESTING PRODUCTS
8.3.4 SEXUALLY TRANSMITTED DISEASES (STD) TESTING
8.3.4.1 NAAT-BASED SYSTEMS
8.3.4.2 NON–NAAT-BASED SYSTEMS
8.3.5 HEPATITIS C TESTING PRODUCTS
8.3.5.1 HCV ANTIBODY TESTS
8.3.5.2 HCV VIRAL LOAD TESTS
8.3.6 INFLUENZA TESTING PRODUCTS
8.3.6.1 TRADITIONAL DIAGNOSTIC TEST
8.3.6.1.1 RAPID INFLUENZA DIAGNOSTIC TEST (RIDT)
8.3.6.1.2 DIRECT FLUORESCENT ANTIBODY TEST (DFAT)
8.3.6.1.3 VIRAL CULTURE
8.3.6.1.4 SEROLOGICAL ASSAY
8.3.6.2 MOLECULAR DIAGNOSTIC ASSAY
8.3.6.2.1 RT-PCR
8.3.6.2.2 LOOP-MEDIATED ISOTHERMAL AMPLIFICATION-BASED ASSAY (LAMP)
8.3.6.2.3 NUCLEIC ACID SEQUENCE-BASED AMPLIFICATION TEST (NASBAT)
8.3.6.2.4 SIMPLE AMPLIFICATION-BASED ASSAY (SAMBA)
8.3.7 HEALTHCARE ASSOCIATED INFECTION (HAI) TESTING
8.3.8 TROPICAL DISEASE TESTING PRODUCTS
8.3.9 OTHER INFECTIOUS DISEASE TESTING PRODUCTS
8.4 CARDIOMETABOLIC MONITORING PRODUCTS
8.4.1 CARDIAC MARKER TESTING PRODUCTS
8.4.1.1 HSTNL
8.4.1.2 BNP
8.4.1.3 D-DIMER
8.4.1.4 CK-MB
8.4.1.5 MYOGLOBIN
8.4.2 BLOOD GAS/ELECTROLYTE TESTING PRODUCTS
8.4.3 BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES
8.4.3.1 CARTRIDGES
8.4.3.2 REAGENTS
8.4.4 BLOOD GAS/ELECTROLYTE TESTING INSTRUMENTS
8.4.4.1 PORTABLE
8.4.4.1.1 COMBINED ANALYZERS
8.4.4.1.2 BLOOD GAS ANALYZERS
8.4.4.1.3 ELECTROLYTE ANALYZERS
8.4.4.2 BENCHTOP
8.4.4.2.1 COMBINED ANALYZERS
8.4.4.2.2 BLOOD GAS ANALYZERS
8.4.4.2.3 ELECTROLYTE ANALYZERS
8.4.5 HBA1C TESTING PRODUCTS
8.4.5.1 HBA1C TESTING INSTRUMENTS
8.4.5.2 HBA1C TESTING CONSUMABLES
8.4.6 POC ANALYZER
8.4.7 ECG DEVICE
8.4.7.1 RESTING ECG DEVICES
8.4.7.2 STRESS ECG DEVICES
8.4.7.3 HOLTER MONITORS
8.5 PREGNANCY AND FERTILITY TESTING PRODUCTS
8.5.1 PREGNANCY TESTING PRODUCTS
8.5.1.1 STRIPS/ DIP STICKS AND CARDS
8.5.1.2 MID STREAM DEVICES
8.5.1.3 CASSETTES
8.5.1.4 DIGITAL DEVICES
8.5.1.5 LINE-INDICATOR DEVICES
8.5.2 FERTILITY TESTING PRODUCTS
8.5.2.1 LUTEINIZING HORMONE (LH) URINE TEST
8.5.2.2 FSH TEST
8.5.2.3 OTHERS
8.6 HAEMATOLOGY TESTING PRODUCTS
8.7 COAGULATION MONITORING PRODUCTS
8.7.1 ANTICOAGULATION MONITORING DEVICES
8.7.1.1 PROTHROMBIN TIME/INTERNATIONAL NORMALIZED RATIO (PT-INR) TESTING DEVICES
8.7.1.2 ACTIVATED CLOTTING TIME (ACT)
8.7.1.3 ACTIVATED PARTIAL THROMBOPLASTIN TIME (APPT)
8.7.2 PLATELET FUNCTION MONITORING DEVICES
8.7.3 VISCOELASTIC COAGULATION MONITORING DEVICES
8.7.3.1 ROTATIONAL THROMBOELASTOMETRY (ROTEM)
8.7.3.2 THROMBOELASTOGRAPHY (TEG)
8.8 DRUG-OF-ABUSE (DOA) TESTING PRODUCTS
8.8.1 DOA ANALYSERS
8.8.1.1 IMMUNOASSAYS
8.8.1.2 CHROMATOGRAPHIC DEVICES
8.8.1.3 BREATH ANALYSERS
8.8.2 RAPID TESTING DEVICES
8.8.2.1 URINE TESTING DEVICES
8.8.2.2 ORAL FLUID TESTING DEVICES
8.8.2.3 OTHERS
8.9 URINALYSIS TESTING PRODUCTS
8.9.1 POC URINE STRIP SELF-TESTING
8.9.2 POC URINE TEST STRIP PROFESSIONAL TESTING
8.1 CHOLESTEROL TESTING PRODUCTS
8.10.1 TESTING KITS
8.10.2 INSTRUMENTS
8.10.2.1 TABLE-TOP ANALYZERS
8.10.2.2 HAND-HELD ANALYZERS
8.11 TUMOR/CANCER MARKER TESTING PRODUCTS
8.12 FECAL OCCULT TESTING PRODUCTS
8.12.1 GUAIAC FOB STOOL TEST
8.12.2 LATERAL FLOW IMMUNO-FOB TEST
8.12.3 IMMUNO-FOB AGGLUTINATION TEST
8.12.4 IMMUNO-FOB ELISA TEST
8.13 OTHERS
9 GSA POINT-OF-CARE TESTING (POCT) MARKET, BY PRESCRIPTION MODE
9.1 OVERVIEW
9.2 OTC TESTING
9.3 PRESCRIPTION-BASED TESTING
10 GSA POINT-OF-CARE TESTING (POCT) MARKET, BY PLATFORM
10.1 OVERVIEW
10.2 LATERAL FLOW ASSAYS/IMMUNOCHROMATOGRAPHY TESTS
10.3 IMMUNOASSAYS
10.4 DIPSTICKS
10.5 MOLECULAR DIAGNOSTICS
10.6 CLINICAL CHEMISTRY ASSAYS
10.7 MICROFLUIDICS
10.8 HEMATOLOGY
10.9 OTHERS
11 GSA POINT-OF-CARE-TESTING (POCT) MARKET, BY APPLICATION
11.1 OVERVIEW
11.2 BLOOD GLUCOSE
11.3 INFECTIOUS DISEASES
11.3.1 COVID-19 TESTING
11.3.2 HIV TESTING
11.3.3 HEPATITIS C TESTING
11.3.4 INFLUENZA TESTING
11.3.5 TUBERCULOSIS TESTING
11.3.6 OTHERS
11.4 VITAL SIGN MONITORING
11.5 CARDIAC MONITORING
11.6 COAGULATION
11.7 HAEMATOLOGY
11.8 NON- INVASIVE SPO2 MONITORING
11.9 BLOOD TRANSFUSION
11.1 NON- INVASIVE PCO2 MONITORING
11.11 WHOLE BLOOD ANALYSIS
11.12 OTHERS
12 GSA POINT-OF-CARE TESTING (POCT) MARKET, BY END USER
12.1 OVERVIEW
12.2 HOSPITALS
12.2.1 PRIVATE
12.2.1.1 TIER 1
12.2.1.2 TIER 2
12.2.1.3 TIER 3
12.2.2 PUBLIC
12.3 HOME CARE
12.4 CLINICS
12.5 LABORATORIES
12.6 DIAGNOSTIC CENTERS
12.7 PATHOLOGY LABS
12.8 AMBULATORY SURGERY CENTERS
12.9 ELDERLY CARE CENTERS
12.1 OTHERS
13 GSA POINT-OF-CARE-TESTING (POCT) MARKET, BY DISTRIBUTION CHANNEL
13.1 OVERVIEW
13.2 DIRECT TENDER
13.3 RETAIL SALES
13.4 ONLINE SALES
13.5 OTHERS
14 BENELUX POINT-OF-CARE TESTING (POCT) MARKET, BY PRODUCT TYPE
14.1 OVERVIEW
14.2 GLUCOSE MONITORING PRODUCTS
14.2.1 STRIPS
14.2.2 METERS
14.2.3 LANCETS AND LANCING DEVICES
14.3 INFECTIOUS DISEASE TESTING PRODUCTS
14.3.1 COVID-19
14.3.2 HIV TESTING PRODUCTS
14.3.2.1 TESTING REAGENTS
14.3.2.2 TESTING EQUIPMENT
14.3.3 RESPIRATORY INFECTION TESTING PRODUCTS
14.3.4 SEXUALLY TRANSMITTED DISEASES (STD) TESTING
14.3.5 NAAT-BASED SYSTEMS
14.3.6 NON–NAAT-BASED SYSTEMS
14.3.7 HEPATITIS C TESTING PRODUCTS
14.3.7.1 HCV ANTIBODY TESTS
14.3.7.2 HCV VIRAL LOAD TESTS
14.3.8 INFLUENZA TESTING PRODUCTS
14.3.8.1 TRADITIONAL DIAGNOSTIC TEST
14.3.8.1.1 RAPID INFLUENZA DIAGNOSTIC TEST (RIDT)
14.3.8.1.2 DIRECT FLUORESCENT ANTIBODY TEST (DFAT)
14.3.8.1.3 VIRAL CULTURE
14.3.8.1.4 SEROLOGICAL ASSAY
14.3.8.2 MOLECULAR DIAGNOSTIC ASSAY
14.3.8.2.1 RT-PCR
14.3.8.2.2 LOOP-MEDIATED ISOTHERMAL AMPLIFICATION-BASED ASSAY (LAMP)
14.3.8.2.3 NUCLEIC ACID SEQUENCE-BASED AMPLIFICATION TEST (NASBAT)
14.3.8.2.4 SIMPLE AMPLIFICATION-BASED ASSAY (SAMBA)
14.3.9 HEALTHCARE ASSOCIATED INFECTION (HAI) TESTING
14.3.10 TROPICAL DISEASE TESTING PRODUCTS
14.3.11 OTHER INFECTIOUS DISEASE TESTING PRODUCTS
14.4 CARDIOMETABOLIC MONITORING PRODUCTS
14.4.1 CARDIAC MARKER TESTING PRODUCTS
14.4.1.1 HSTNL
14.4.1.2 BNP
14.4.1.3 D-DIMER
14.4.1.4 CK-MB
14.4.1.5 MYOGLOBIN
14.4.2 BLOOD GAS/ELECTROLYTE TESTING PRODUCTS
14.4.3 BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES
14.4.3.1 CARTRIDGES
14.4.3.2 REAGENTS
14.4.4 BLOOD GAS/ELECTROLYTE TESTING INSTRUMENTS
14.4.5 PORTABLE
14.4.5.1 COMBINED ANALYZERS
14.4.5.2 BLOOD GAS ANALYZERS
14.4.5.3 ELECTROLYTE ANALYZERS
14.4.6 BENCHTOP
14.4.6.1 COMBINED ANALYZERS
14.4.6.2 BLOOD GAS ANALYZERS
14.4.6.3 ELECTROLYTE ANALYZERS
14.4.7 HBA1C TESTING PRODUCTS
14.4.7.1 HBA1C TESTING INSTRUMENTS
14.4.7.2 HBA1C TESTING CONSUMABLES
14.4.8 POC ANALYZER
14.4.9 ECG DEVICE
14.4.9.1 RESTING ECG DEVICES
14.4.9.2 STRESS ECG DEVICES
14.4.9.3 HOLTER MONITORS
14.5 PREGNANCY AND FERTILITY TESTING PRODUCTS
14.5.1 PREGNANCY TESTING PRODUCTS
14.5.1.1 STRIPS/ DIP STICKS AND CARDS
14.5.1.2 MID STREAM DEVICES
14.5.1.3 CASSETTES
14.5.1.4 DIGITAL DEVICES
14.5.1.5 LINE-INDICATOR DEVICES
14.5.2 FERTILITY TESTING PRODUCTS
14.5.2.1 LUTEINIZING HORMONE (LH) URINE TEST
14.5.2.2 FSH TEST
14.5.2.3 OTHERS
14.6 HAEMATOLOGY TESTING PRODUCTS
14.7 COAGULATION MONITORING PRODUCTS
14.8 ANTICOAGULATION MONITORING DEVICES
14.8.1 PROTHROMBIN TIME/INTERNATIONAL NORMALIZED RATIO (PT-INR) TESTING DEVICES
14.8.2 ACTIVATED CLOTTING TIME (ACT)
14.8.3 ACTIVATED PARTIAL THROMBOPLASTIN TIME (APPT)
14.8.4 PLATELET FUNCTION MONITORING DEVICES
14.8.5 VISCOELASTIC COAGULATION MONITORING DEVICES
14.8.5.1 ROTATIONAL THROMBOELASTOMETRY (ROTEM)
14.8.5.2 THROMBOELASTOGRAPHY (TEG)
14.9 DRUG-OF-ABUSE (DOA) TESTING PRODUCTS
14.9.1 DOA ANALYSERS
14.9.1.1 IMMUNOASSAYS
14.9.1.2 CHROMATOGRAPHIC DEVICES
14.9.1.3 BREATH ANALYSERS
14.9.2 RAPID TESTING DEVICES
14.9.2.1 URINE TESTING DEVICES
14.9.2.2 ORAL FLUID TESTING DEVICES
14.9.3 OTHERS
14.1 URINALYSIS TESTING PRODUCTS
14.10.1 POC URINE STRIP SELF-TESTING
14.10.2 POC URINE TEST STRIP PROFESSIONAL TESTING
14.11 CHOLESTEROL TESTING PRODUCTS
14.11.1 TESTING KITS
14.11.2 INSTRUMENTS
14.11.2.1 TABLE-TOP ANALYZERS
14.11.2.2 HAND-HELD ANALYZERS
14.12 TUMOR/CANCER MARKER TESTING PRODUCTS
14.13 FECAL OCCULT TESTING PRODUCTS
14.13.1 GUAIAC FOB STOOL TEST
14.13.2 LATERAL FLOW IMMUNO-FOB TEST
14.13.3 IMMUNO-FOB AGGLUTINATION TEST
14.13.4 IMMUNO-FOB ELISA TEST
14.14 OTHERS
15 BENELUX POINT-OF-CARE TESTING (POCT) MARKET, BY PLATFORM
15.1 OVERVIEW
15.2 LATERAL FLOW ASSAYS/IMMUNOCHROMATOGRAPHY TESTS
15.3 IMMUNOASSAYS
15.4 DIPSTICKS
15.5 MOLECULAR DIAGNOSTICS
15.6 CLINICAL CHEMISTRY ASSAYS
15.7 MICROFLUIDICS
15.8 HEMATOLOGY
15.9 OTHERS
16 BENELUX POINT-OF-CARE TESTING (POCT) MARKET, BY PRESCRIPTION MODE
16.1 OVERVIEW
16.2 OTC TESTING
16.3 PRESCRIPTION-BASED TESTING
17 BENELUX POINT-OF-CARE-TESTING (POCT) MARKET, BY APPLICATION
17.1 OVERVIEW
17.2 BLOOD GLUCOSE
17.3 INFECTIOUS DISEASES
17.3.1.1 COVID-19 TESTING
17.3.1.2 HIV TESTING
17.3.1.3 HEPATITIS C TESTING
17.3.1.4 INFLUENZA TESTING
17.3.1.5 TUBERCULOSIS TESTING
17.3.1.6 OTHERS
17.4 VITAL SIGN MONITORING
17.5 CARDIAC MONITORING
17.6 COAGULATION
17.7 HAEMATOLOGY
17.8 NON- INVASIVE SPO2 MONITORING
17.9 BLOOD TRANSFUSION
17.1 NON- INVASIVE PCO2 MONITORING
17.11 WHOLE BLOOD ANALYSIS
17.12 OTHERS
18 BENELUX POINT-OF-CARE TESTING (POCT) MARKET, BY END USER
18.1 OVERVIEW
18.2 HOSPITALS
18.2.1 PRIVATE
18.2.1.1 TIER 1
18.2.1.2 TIER 2
18.2.1.3 TIER 3
18.2.2 PUBLIC
18.3 HOME CARE
18.4 CLINICS
18.5 LABORATORIES
18.6 DIAGNOSTIC CENTERS
18.7 PATHOLOGY LABS
18.8 AMBULATORY SURGERY CENTERS
18.9 ELDERLY CARE CENTERS
18.1 OTHERS
19 BENELUX POINT-OF-CARE-TESTING (POCT) MARKET, BY DISTRIBUTION CHANNEL
19.1 OVERVIEW
19.2 DIRECT TENDER
19.3 RETAIL SALES
19.4 ONLINE SALES
19.5 OTHERS
20 GSA & BENELUX
20.1 GERMANY
20.2 NETHERLANDS
20.3 SWITZERLAND
20.4 BELGIUM
20.5 AUSTRIA
20.6 LUXEMBOURG
21 GSA AND BENELUX POINT-OF-CARE-TESTING (POCT) MARKET: COMPANY LANDSCAPE
21.1 COMPANY SHARE ANALYSIS: GSA AND BENELUX
22 SWOT ANALYSIS
23 COMPANY PROFILE
23.1 F. HOFFMANN-LA ROCHE LTD
23.1.1 COMPANY SNAPSHOT
23.1.2 REVENUE ANALYSIS
23.1.3 PRODUCT PORTFOLIO
23.1.4 RECENT DEVELOPMENT
23.2 DANAHER CORPORATION
23.2.1 COMPANY SNAPSHOT
23.2.2 REVENUE ANALYSIS
23.2.3 PRODUCT PORTFOLIO
23.2.4 RECENT DEVELOPMENT
23.3 SIEMENS HEALTHINEERS AG
23.3.1 COMPANY SNAPSHOT
23.3.2 REVENUE ANALYSIS
23.3.3 PRODUCT PORTFOLIO
23.3.4 RECENT DEVELOPMENT
23.4 BIO- RAD LABORATORIES, INC.
23.4.1 COMPANY SNAPSHOT
23.4.2 REVENUE ANALYSIS
23.4.3 PRODUCT PORTFOLIO
23.4.4 RECENT DEVELOPMENT
23.5 BIOMERIEUX
23.5.1 COMPANY SNAPSHOT
23.5.2 REVENUE ANALYSIS
23.5.3 PRODUCT PORTFOLIO
23.5.4 RECENT DEVELOPMENT
23.6 ACCUBIOTECH CO., LTD.
23.6.1 COMPANY SNAPSHOT
23.6.2 PRODUCT PORTFOLIO
23.6.3 RECENT DEVELOPMENT
23.7 ABBOTT
23.7.1 COMPANY SNAPSHOT
23.7.2 REVENUE ANALYSIS
23.7.3 PRODUCT PORTFOLIO
23.7.4 RECENT DEVELOPMENT/NEWS
23.8 BINX HEALTH
23.8.1 COMPANY SNAPSHOT
23.8.2 SOLUTION PORTFOLIO
23.8.3 RECENT DEVELOPMENT
23.9 BD
23.9.1 COMPANY SNAPSHOT
23.9.2 REVENUE ANALYSIS
23.9.3 PRODUCT PORTFOLIO
23.9.4 RECENT DEVELOPMENT/NEWS
23.1 EKF DIAGNOSTICS HOLDINGS PLC
23.10.1 COMPANY SNAPSHOT
23.10.2 REVENUE ANALYSIS
23.10.3 PRODUCT PORTFOLIO
23.10.4 RECENT DEVELOPMENT
23.11 HOLOGIC, INC
23.11.1 COMPANY SNAPSHOT
23.11.2 REVENUE ANALYSIS
23.11.3 PRODUCT PORTFOLIO
23.11.4 RECENT DEVELOPMENT
23.12 MERIDIAN BIOSCIENCE, INC.
23.12.1 COMPANY SNAPSHOT
23.12.2 PRODUCT PORTFOLIO
23.12.3 RECENT DEVELOPMENT/NEWS
23.13 QIAGEN
23.13.1 COMPANY SNAPSHOT
23.13.2 REVENUE ANALYSIS
23.13.3 PRODUCT PORTFOLIO
23.13.4 RECENT DEVELOPMENT
23.14 QUIDELORTHO CORPORATION
23.14.1 COMPANY SNAPSHOT
23.14.2 REVENUE ANALYSIS
23.14.3 PRODUCT PORTFOLIO
23.14.4 RECENT DEVELOPMENT
23.15 SEKISUI DIAGNOSTICS
23.15.1 COMPANY SNAPSHOT
23.15.2 PRODUCT PORTFOLIO
23.15.3 RECENT DEVELOPMENT
23.16 SYSMEX CORPORATION
23.16.1 COMPANY SNAPSHOT
23.16.2 REVENUE ANALYSIS
23.16.3 PRODUCT PORTFOLIO
23.16.4 RECENT DEVELOPMENT
23.17 SIENCO, INC.
23.17.1 COMPANY SNAPSHOT
23.17.2 PRODUCT PORTFOLIO
23.17.3 RECENT DEVELOPMENT
23.18 TRINITY BIOTECH
23.18.1 COMPANY SNAPSHOT
23.18.2 REVENUE ANALYSIS
23.18.3 PRODUCT PORTFOLIO
23.18.4 RECENT DEVELOPMENT
23.19 THERMO FISHER SCIENTIFIC INC.
23.19.1 COMPANY SNAPSHOT
23.19.2 REVENUE ANALYSIS
23.19.3 PRODUCT PORTFOLIO
23.19.4 RECENT DEVELOPMENT
23.2 WERFEN
23.20.1 COMPANY SNAPSHOT
23.20.2 PRODUCT PORTFOLIO
23.20.3 RECENT DEVELOPMENT
24 QUESTIONNAIRE
Liste des tableaux
TABLE 1 HOME-USE POCT DEVICES WITH TELEMEDICINE INTEGRATION
TABLE 2 COMPANIES AND PRODUCTS WITH TELEMEDICINE INTEGRATION
TABLE 3 A TECHNOLOGY ROADMAP OUTLINING THE FUTURE OF CONNECTED POCT DEVICES
TABLE 4 GSA POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 5 GSA POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 6 GSA POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 7 GSA GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 8 GSA GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 9 GSA GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 10 GSA INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 11 GSA INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 12 GSA INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 13 GSA HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 14 GSA HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 15 GSA HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 16 GSA SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 17 GSA SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 18 GSA SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 19 GSA HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 20 GSA HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 21 GSA HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 22 GSA INFLUENZA TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 23 GSA TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 24 GSA TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 25 GSA TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 26 GSA MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 27 GSA MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 28 GSA MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 29 GSA CARDIOMETABOLIC MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 30 GSA POC ANALYZER IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 31 GSA POC ANALYZER IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 32 GSA CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 33 GSA CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 34 GSA CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 35 GSA BLOOD GAS/ELECTROLYTE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 36 GSA BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 37 GSA BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 38 GSA BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 39 GSA BLOOD GAS/ELECTROLYTE TESTING INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 40 GSA PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 41 GSA PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 42 GSA PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 43 GSA BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 44 GSA BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 45 GSA BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 46 GSA HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 47 GSA HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 48 GSA HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 49 GSA ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 50 GSA ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 51 GSA ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 52 GSA PREGNANCY AND FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 53 GSA PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 54 GSA PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 55 GSA PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 56 GSA FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 57 GSA FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 58 GSA FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 59 GSA COAGULATION MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 60 GSA ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 61 GSA ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 62 GSA ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 63 GSA VISCOELASTIC COAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY METHOD, 2018-2032 (USD THOUSAND)
TABLE 64 GSA DRUG-OF-ABUSE (DOA) TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 65 GSA DOA ANALYZERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 66 GSA DOA ANALYZERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNIT)
TABLE 67 GSA DOA ANALYZERS MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 68 GSA RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 69 GSA RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 70 GSA RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 71 GSA URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 72 GSA URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 73 GSA URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 74 GSA CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 75 GSA CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 76 GSA CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 77 GSA INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 78 GSA INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 79 GSA INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 80 GSA FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 81 GSA FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 82 GSA FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 83 GSA POINT-OF-CARE-TESTING (POCT) MARKET, BY PRESCRIPTION MODE, 2018-2032 (USD THOUSAND)
TABLE 84 GSA POINT-OF-CARE-TESTING (POCT) MARKET, BY PLATFORM, 2018-2032 (USD THOUSAND)
TABLE 85 GSA POINT-OF-CARE-TESTING (POCT) MARKET, BY APPLICATION, 2018-2032 (USD THOUSAND)
TABLE 86 GSA INFECTIOUS DISEASES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 87 GSA POINT-OF-CARE-TESTING (POCT) MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 88 GSA HOSPITALS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 89 GSA PRIVATE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY LEVEL, 2018-2032 (USD THOUSAND)
TABLE 90 GSA PUBLIC IN POINT-OF-CARE-TESTING (POCT) MARKET, BY LEVEL, 2018-2032 (USD THOUSAND)
TABLE 91 GSA POINT-OF-CARE-TESTING (POCT) MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 92 BENELUX POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 93 BENELUX POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 94 BENELUX POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 95 BENELUX GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 96 BENELUX GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 97 BENELUX GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 98 BENELUX INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 99 BENELUX INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 100 BENELUX INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 101 BENELUX HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 102 BENELUX HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 103 BENELUX HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 104 BENELUX SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 105 BENELUX SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 106 BENELUX SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 107 BENELUX HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 108 BENELUX HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 109 BENELUX HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 110 BENELUX INFLUENZA TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 111 BENELUX TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 112 BENELUX TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 113 BENELUX TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 114 BENELUX MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 115 BENELUX MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 116 BENELUX MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 117 BENELUX CARDIOMETABOLIC MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 118 BENELUX POC ANALYZER IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 119 BENELUX POC ANALYZER IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 120 BENELUX CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 121 BENELUX CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 122 BENELUX CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 123 BENELUX BLOOD GAS/ELECTROLYTE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 124 BENELUX BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 125 BENELUX BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 126 BENELUX BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 127 BENELUX BLOOD GAS/ELECTROLYTE TESTING INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 128 BENELUX PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 129 BENELUX PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 130 BENELUX PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 131 BENELUX BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 132 BENELUX BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 133 BENELUX BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 134 BENELUX HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 135 BENELUX HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 136 BENELUX HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 137 BENELUX ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 138 BENELUX ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 139 BENELUX ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 140 BENELUX PREGNANCY AND FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 141 BENELUX PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 142 BENELUX PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 143 BENELUX PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 144 BENELUX FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 145 BENELUX FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 146 BENELUX FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 147 BENELUX COAGULATION MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 148 BENELUX ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 149 BENELUX ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 150 BENELUX ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 151 BENELUX VISCOELASTIC COAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY METHOD, 2018-2032 (USD THOUSAND)
TABLE 152 BENELUX DRUG-OF-ABUSE (DOA) TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 153 BENELUX DOA ANALYZERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 154 BENELUX DOA ANALYZERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNIT)
TABLE 155 BENELUX DOA ANALYZERS MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 156 BENELUX RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 157 BENELUX RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 158 BENELUX RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 159 BENELUX URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 160 BENELUX URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 161 BENELUX URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 162 BENELUX CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 163 BENELUX CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 164 BENELUX CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 165 BENELUX INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 166 BENELUX INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 167 BENELUX INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 168 BENELUX FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 169 BENELUX FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 170 BENELUX FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 171 BENELUX POINT-OF-CARE-TESTING (POCT) MARKET, BY PLATFORM, 2018-2032 (USD THOUSAND)
TABLE 172 BENELUX POINT-OF-CARE-TESTING (POCT) MARKET, BY PRESCRIPTION MODE, 2018-2032 (USD THOUSAND)
TABLE 173 BENELUX POINT-OF-CARE-TESTING (POCT) MARKET, BY APPLICATION, 2018-2032 (USD THOUSAND)
TABLE 174 BENELUX INFECTIOUS DISEASES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 175 BENELUX POINT-OF-CARE-TESTING (POCT) MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 176 BENELUX HOSPITALS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 177 BENELUX PRIVATE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY LEVEL, 2018-2032 (USD THOUSAND)
TABLE 178 BENELUX PUBLIC IN POINT-OF-CARE-TESTING (POCT) MARKET, BY LEVEL, 2018-2032 (USD THOUSAND)
TABLE 179 BENELUX POINT-OF-CARE-TESTING (POCT) MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 180 GSA & BENELUX POINT-OF-CARE-TESTING (POCT) MARKET, BY COUNTRY, 2018-2032 (USD THOUSAND)
TABLE 181 BENELUX POINT-OF-CARE-TESTING (POCT) MARKET, BY COUNTRY, 2018-2032 (USD THOUSAND)
TABLE 182 GSA POINT-OF-CARE-TESTING (POCT) MARKET, BY COUNTRY, 2018-2032 (USD THOUSAND)
TABLE 183 GERMANY POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 184 GERMANY POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 185 GERMANY POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 186 GERMANY GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 187 GERMANY GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 188 GERMANY GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 189 GERMANY INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 190 GERMANY INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 191 GERMANY INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 192 GERMANY HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 193 GERMANY HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 194 GERMANY HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 195 GERMANY SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 196 GERMANY SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 197 GERMANY SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 198 GERMANY HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 199 GERMANY HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 200 GERMANY HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 201 GERMANY INFLUENZA TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 202 GERMANY TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 203 GERMANY TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 204 GERMANY TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 205 GERMANY MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 206 GERMANY MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 207 GERMANY MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 208 GERMANY CARDIOMETABOLIC MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 209 GERMANY POC ANALYZER IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 210 GERMANY POC ANALYZER IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 211 GERMANY CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 212 GERMANY CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 213 GERMANY CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 214 GERMANY BLOOD GAS/ELECTROLYTE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 215 GERMANY BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 216 GERMANY BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 217 GERMANY BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 218 GERMANY BLOOD GAS/ELECTROLYTE TESTING INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 219 GERMANY PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 220 GERMANY PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 221 GERMANY PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 222 GERMANY BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 223 GERMANY BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 224 GERMANY BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 225 GERMANY HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 226 GERMANY HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 227 GERMANY HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 228 GERMANY ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 229 GERMANY ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 230 GERMANY ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 231 GERMANY PREGNANCY AND FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 232 GERMANY PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 233 GERMANY PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 234 GERMANY PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 235 GERMANY FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 236 GERMANY FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 237 GERMANY FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 238 GERMANY COAGULATION MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 239 GERMANY ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 240 GERMANY ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 241 GERMANY ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 242 GERMANY VISCOELASTIC COAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY METHOD, 2018-2032 (USD THOUSAND)
TABLE 243 GERMANY DRUG-OF-ABUSE (DOA) TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 244 GERMANY DOA ANALYZERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 245 GERMANY DOA ANALYZERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 246 GERMANY DOA ANALYZERS MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 247 GERMANY RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 248 GERMANY RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 249 GERMANY RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 250 GERMANY URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 251 GERMANY URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 252 GERMANY URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 253 GERMANY CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 254 GERMANY CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 255 GERMANY CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 256 GERMANY INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 257 GERMANY INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 258 GERMANY INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 259 GERMANY FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 260 GERMANY FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 261 GERMANY FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 262 GERMANY POINT-OF-CARE-TESTING (POCT) MARKET, BY PLATFORM, 2018-2032 (USD THOUSAND)
TABLE 263 GERMANY POINT-OF-CARE-TESTING (POCT) MARKET, BY APPLICATION, 2018-2032 (USD THOUSAND)
TABLE 264 GERMANY INFECTIOUS DISEASES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 265 GERMANY POINT-OF-CARE-TESTING (POCT) MARKET, BY PRESCRIPTION MODE, 2018-2032 (USD THOUSAND)
TABLE 266 GERMANY POINT-OF-CARE-TESTING (POCT) MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 267 GERMANY HOSPITALS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 268 GERMANY PRIVATE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY LEVEL, 2018-2032 (USD THOUSAND)
TABLE 269 GERMANY PUBLIC IN POINT-OF-CARE-TESTING (POCT) MARKET, BY LEVEL, 2018-2032 (USD THOUSAND)
TABLE 270 GERMANY POINT-OF-CARE-TESTING (POCT) MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 271 NETHERLANDS POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 272 NETHERLANDS POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 273 NETHERLANDS POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 274 NETHERLANDS GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 275 NETHERLANDS GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 276 NETHERLANDS GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 277 NETHERLANDS INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 278 NETHERLANDS INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 279 NETHERLANDS INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 280 NETHERLANDS HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 281 NETHERLANDS HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 282 NETHERLANDS HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 283 NETHERLANDS SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 284 NETHERLANDS SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 285 NETHERLANDS SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 286 NETHERLANDS HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 287 NETHERLANDS HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 288 NETHERLANDS HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 289 NETHERLANDS INFLUENZA TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 290 NETHERLANDS TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 291 NETHERLANDS TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 292 NETHERLANDS TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 293 NETHERLANDS MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 294 NETHERLANDS MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 295 NETHERLANDS MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 296 NETHERLANDS CARDIOMETABOLIC MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 297 NETHERLANDS POC ANALYZER IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 298 NETHERLANDS POC ANALYZER IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 299 NETHERLANDS CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 300 NETHERLANDS CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 301 NETHERLANDS CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 302 NETHERLANDS BLOOD GAS/ELECTROLYTE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 303 NETHERLANDS BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 304 NETHERLANDS BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 305 NETHERLANDS BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 306 NETHERLANDS BLOOD GAS/ELECTROLYTE TESTING INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 307 NETHERLANDS PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 308 NETHERLANDS PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 309 NETHERLANDS PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 310 NETHERLANDS BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 311 NETHERLANDS BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 312 NETHERLANDS BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 313 NETHERLANDS HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 314 NETHERLANDS HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 315 NETHERLANDS HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 316 NETHERLANDS ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 317 NETHERLANDS ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 318 NETHERLANDS ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 319 NETHERLANDS PREGNANCY AND FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 320 NETHERLANDS PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 321 NETHERLANDS PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 322 NETHERLANDS PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 323 NETHERLANDS FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 324 NETHERLANDS FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 325 NETHERLANDS FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 326 NETHERLANDS COAGULATION MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 327 NETHERLANDS ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 328 NETHERLANDS ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 329 NETHERLANDS ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 330 NETHERLANDS VISCOELASTIC COAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY METHOD, 2018-2032 (USD THOUSAND)
TABLE 331 NETHERLANDS DRUG-OF-ABUSE (DOA) TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 332 NETHERLANDS DOA ANALYZERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 333 NETHERLANDS DOA ANALYZERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 334 NETHERLANDS DOA ANALYZERS MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 335 NETHERLANDS RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 336 NETHERLANDS RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 337 NETHERLANDS RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 338 NETHERLANDS URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 339 NETHERLANDS URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 340 NETHERLANDS URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 341 NETHERLANDS CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 342 NETHERLANDS CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 343 NETHERLANDS CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 344 NETHERLANDS INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 345 NETHERLANDS INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 346 NETHERLANDS INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 347 NETHERLANDS FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 348 NETHERLANDS FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 349 NETHERLANDS FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 350 NETHERLANDS POINT-OF-CARE-TESTING (POCT) MARKET, BY PLATFORM, 2018-2032 (USD THOUSAND)
TABLE 351 NETHERLANDS POINT-OF-CARE-TESTING (POCT) MARKET, BY APPLICATION, 2018-2032 (USD THOUSAND)
TABLE 352 NETHERLANDS INFECTIOUS DISEASES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 353 NETHERLANDS POINT-OF-CARE-TESTING (POCT) MARKET, BY PRESCRIPTION MODE, 2018-2032 (USD THOUSAND)
TABLE 354 NETHERLANDS POINT-OF-CARE-TESTING (POCT) MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 355 NETHERLANDS HOSPITALS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 356 NETHERLANDS PRIVATE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY LEVEL, 2018-2032 (USD THOUSAND)
TABLE 357 NETHERLANDS PUBLIC IN POINT-OF-CARE-TESTING (POCT) MARKET, BY LEVEL, 2018-2032 (USD THOUSAND)
TABLE 358 NETHERLANDS POINT-OF-CARE-TESTING (POCT) MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 359 SWITZERLAND POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 360 SWITZERLAND POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 361 SWITZERLAND POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 362 SWITZERLAND GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 363 SWITZERLAND GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 364 SWITZERLAND GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 365 SWITZERLAND INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 366 SWITZERLAND INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 367 SWITZERLAND INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 368 SWITZERLAND HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 369 SWITZERLAND HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 370 SWITZERLAND HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 371 SWITZERLAND SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 372 SWITZERLAND SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 373 SWITZERLAND SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 374 SWITZERLAND HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 375 SWITZERLAND HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 376 SWITZERLAND HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 377 SWITZERLAND INFLUENZA TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 378 SWITZERLAND TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 379 SWITZERLAND TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 380 SWITZERLAND TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 381 SWITZERLAND MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 382 SWITZERLAND MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 383 SWITZERLAND MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 384 SWITZERLAND CARDIOMETABOLIC MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 385 SWITZERLAND POC ANALYZER IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 386 SWITZERLAND POC ANALYZER IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 387 SWITZERLAND CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 388 SWITZERLAND CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 389 SWITZERLAND CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 390 SWITZERLAND BLOOD GAS/ELECTROLYTE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 391 SWITZERLAND BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 392 SWITZERLAND BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 393 SWITZERLAND BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 394 SWITZERLAND BLOOD GAS/ELECTROLYTE TESTING INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 395 SWITZERLAND PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 396 SWITZERLAND PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 397 SWITZERLAND PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 398 SWITZERLAND BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 399 SWITZERLAND BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 400 SWITZERLAND BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 401 SWITZERLAND HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 402 SWITZERLAND HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 403 SWITZERLAND HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 404 SWITZERLAND ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 405 SWITZERLAND ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 406 SWITZERLAND ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 407 SWITZERLAND PREGNANCY AND FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 408 SWITZERLAND PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 409 SWITZERLAND PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 410 SWITZERLAND PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 411 SWITZERLAND FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 412 SWITZERLAND FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 413 SWITZERLAND FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 414 SWITZERLAND COAGULATION MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 415 SWITZERLAND ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 416 SWITZERLAND ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 417 SWITZERLAND ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 418 SWITZERLAND VISCOELASTIC COAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY METHOD, 2018-2032 (USD THOUSAND)
TABLE 419 SWITZERLAND DRUG-OF-ABUSE (DOA) TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 420 SWITZERLAND DOA ANALYZERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 421 SWITZERLAND DOA ANALYZERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 422 SWITZERLAND DOA ANALYZERS MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 423 SWITZERLAND RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 424 SWITZERLAND RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 425 SWITZERLAND RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 426 SWITZERLAND URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 427 SWITZERLAND URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 428 SWITZERLAND URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 429 SWITZERLAND CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 430 SWITZERLAND CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 431 SWITZERLAND CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 432 SWITZERLAND INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 433 SWITZERLAND INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 434 SWITZERLAND INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 435 SWITZERLAND FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 436 SWITZERLAND FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 437 SWITZERLAND FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 438 SWITZERLAND POINT-OF-CARE-TESTING (POCT) MARKET, BY PLATFORM, 2018-2032 (USD THOUSAND)
TABLE 439 SWITZERLAND POINT-OF-CARE-TESTING (POCT) MARKET, BY APPLICATION, 2018-2032 (USD THOUSAND)
TABLE 440 SWITZERLAND INFECTIOUS DISEASES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 441 SWITZERLAND POINT-OF-CARE-TESTING (POCT) MARKET, BY PRESCRIPTION MODE, 2018-2032 (USD THOUSAND)
TABLE 442 SWITZERLAND POINT-OF-CARE-TESTING (POCT) MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 443 SWITZERLAND HOSPITALS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 444 SWITZERLAND PRIVATE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY LEVEL, 2018-2032 (USD THOUSAND)
TABLE 445 SWITZERLAND PUBLIC IN POINT-OF-CARE-TESTING (POCT) MARKET, BY LEVEL, 2018-2032 (USD THOUSAND)
TABLE 446 SWITZERLAND POINT-OF-CARE-TESTING (POCT) MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 447 BELGIUM POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 448 BELGIUM POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 449 BELGIUM POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 450 BELGIUM GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 451 BELGIUM GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 452 BELGIUM GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 453 BELGIUM INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 454 BELGIUM INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 455 BELGIUM INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 456 BELGIUM HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 457 BELGIUM HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 458 BELGIUM HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 459 BELGIUM SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 460 BELGIUM SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 461 BELGIUM SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 462 BELGIUM HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 463 BELGIUM HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 464 BELGIUM HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 465 BELGIUM INFLUENZA TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 466 BELGIUM TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 467 BELGIUM TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 468 BELGIUM TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 469 BELGIUM MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 470 BELGIUM MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 471 BELGIUM MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 472 BELGIUM CARDIOMETABOLIC MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 473 BELGIUM POC ANALYZER IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 474 BELGIUM POC ANALYZER IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 475 BELGIUM CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 476 BELGIUM CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 477 BELGIUM CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 478 BELGIUM BLOOD GAS/ELECTROLYTE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 479 BELGIUM BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 480 BELGIUM BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 481 BELGIUM BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 482 BELGIUM BLOOD GAS/ELECTROLYTE TESTING INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 483 BELGIUM PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 484 BELGIUM PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 485 BELGIUM PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 486 BELGIUM BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 487 BELGIUM BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 488 BELGIUM BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 489 BELGIUM HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 490 BELGIUM HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 491 BELGIUM HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 492 BELGIUM ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 493 BELGIUM ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 494 BELGIUM ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 495 BELGIUM PREGNANCY AND FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 496 BELGIUM PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 497 BELGIUM PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 498 BELGIUM PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 499 BELGIUM FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 500 BELGIUM FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 501 BELGIUM FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 502 BELGIUM COAGULATION MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 503 BELGIUM ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 504 BELGIUM ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 505 BELGIUM ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 506 BELGIUM VISCOELASTIC COAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY METHOD, 2018-2032 (USD THOUSAND)
TABLE 507 BELGIUM DRUG-OF-ABUSE (DOA) TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 508 BELGIUM DOA ANALYZERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 509 BELGIUM DOA ANALYZERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 510 BELGIUM DOA ANALYZERS MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 511 BELGIUM RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 512 BELGIUM RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 513 BELGIUM RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 514 BELGIUM URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 515 BELGIUM URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 516 BELGIUM URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 517 BELGIUM CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 518 BELGIUM CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 519 BELGIUM CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 520 BELGIUM INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 521 BELGIUM INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 522 BELGIUM INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 523 BELGIUM FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 524 BELGIUM FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 525 BELGIUM FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 526 BELGIUM POINT-OF-CARE-TESTING (POCT) MARKET, BY PLATFORM, 2018-2032 (USD THOUSAND)
TABLE 527 BELGIUM POINT-OF-CARE-TESTING (POCT) MARKET, BY APPLICATION, 2018-2032 (USD THOUSAND)
TABLE 528 BELGIUM INFECTIOUS DISEASES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 529 BELGIUM POINT-OF-CARE-TESTING (POCT) MARKET, BY PRESCRIPTION MODE, 2018-2032 (USD THOUSAND)
TABLE 530 BELGIUM POINT-OF-CARE-TESTING (POCT) MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 531 BELGIUM HOSPITALS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 532 BELGIUM PRIVATE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY LEVEL, 2018-2032 (USD THOUSAND)
TABLE 533 BELGIUM PUBLIC IN POINT-OF-CARE-TESTING (POCT) MARKET, BY LEVEL, 2018-2032 (USD THOUSAND)
TABLE 534 BELGIUM POINT-OF-CARE-TESTING (POCT) MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 535 AUSTRIA POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 536 AUSTRIA POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 537 AUSTRIA POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 538 AUSTRIA GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 539 AUSTRIA GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 540 AUSTRIA GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 541 AUSTRIA INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 542 AUSTRIA INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 543 AUSTRIA INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 544 AUSTRIA HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 545 AUSTRIA HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 546 AUSTRIA HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 547 AUSTRIA SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 548 AUSTRIA SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 549 AUSTRIA SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 550 AUSTRIA HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 551 AUSTRIA HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 552 AUSTRIA HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 553 AUSTRIA INFLUENZA TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 554 AUSTRIA TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 555 AUSTRIA TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 556 AUSTRIA TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 557 AUSTRIA MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 558 AUSTRIA MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 559 AUSTRIA MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 560 AUSTRIA CARDIOMETABOLIC MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 561 AUSTRIA POC ANALYZER IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 562 AUSTRIA POC ANALYZER IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 563 AUSTRIA CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 564 AUSTRIA CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 565 AUSTRIA CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 566 AUSTRIA BLOOD GAS/ELECTROLYTE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 567 AUSTRIA BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 568 AUSTRIA BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 569 AUSTRIA BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 570 AUSTRIA BLOOD GAS/ELECTROLYTE TESTING INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 571 AUSTRIA PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 572 AUSTRIA PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 573 AUSTRIA PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 574 AUSTRIA BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 575 AUSTRIA BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 576 AUSTRIA BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 577 AUSTRIA HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 578 AUSTRIA HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 579 AUSTRIA HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 580 AUSTRIA ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 581 AUSTRIA ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 582 AUSTRIA ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 583 AUSTRIA PREGNANCY AND FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 584 AUSTRIA PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 585 AUSTRIA PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 586 AUSTRIA PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 587 AUSTRIA FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 588 AUSTRIA FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 589 AUSTRIA FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 590 AUSTRIA COAGULATION MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 591 AUSTRIA ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 592 AUSTRIA ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 593 AUSTRIA ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 594 AUSTRIA VISCOELASTIC COAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY METHOD, 2018-2032 (USD THOUSAND)
TABLE 595 AUSTRIA DRUG-OF-ABUSE (DOA) TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 596 AUSTRIA DOA ANALYZERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 597 AUSTRIA DOA ANALYZERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 598 AUSTRIA DOA ANALYZERS MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 599 AUSTRIA RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 600 AUSTRIA RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 601 AUSTRIA RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 602 AUSTRIA URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 603 AUSTRIA URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 604 AUSTRIA URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 605 AUSTRIA CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 606 AUSTRIA CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 607 AUSTRIA CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 608 AUSTRIA INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 609 AUSTRIA INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 610 AUSTRIA INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 611 AUSTRIA FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 612 AUSTRIA FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 613 AUSTRIA FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 614 AUSTRIA POINT-OF-CARE-TESTING (POCT) MARKET, BY PLATFORM, 2018-2032 (USD THOUSAND)
TABLE 615 AUSTRIA POINT-OF-CARE-TESTING (POCT) MARKET, BY APPLICATION, 2018-2032 (USD THOUSAND)
TABLE 616 AUSTRIA INFECTIOUS DISEASES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 617 AUSTRIA POINT-OF-CARE-TESTING (POCT) MARKET, BY PRESCRIPTION MODE, 2018-2032 (USD THOUSAND)
TABLE 618 AUSTRIA POINT-OF-CARE-TESTING (POCT) MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 619 AUSTRIA HOSPITALS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 620 AUSTRIA PRIVATE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY LEVEL, 2018-2032 (USD THOUSAND)
TABLE 621 AUSTRIA PUBLIC IN POINT-OF-CARE-TESTING (POCT) MARKET, BY LEVEL, 2018-2032 (USD THOUSAND)
TABLE 622 AUSTRIA POINT-OF-CARE-TESTING (POCT) MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 623 LUXEMBOURG POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 624 LUXEMBOURG POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 625 LUXEMBOURG POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 626 LUXEMBOURG GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 627 LUXEMBOURG GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 628 LUXEMBOURG GLUCOSE MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 629 LUXEMBOURG INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 630 LUXEMBOURG INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 631 LUXEMBOURG INFECTIOUS DISEASE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 632 LUXEMBOURG HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 633 LUXEMBOURG HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 634 LUXEMBOURG HIV TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 635 LUXEMBOURG SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 636 LUXEMBOURG SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 637 LUXEMBOURG SEXUALLY TRANSMITTED DISEASES (STD) TESTING IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 638 LUXEMBOURG HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 639 LUXEMBOURG HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 640 LUXEMBOURG HEPATITIS C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 641 LUXEMBOURG INFLUENZA TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 642 LUXEMBOURG TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 643 LUXEMBOURG TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 644 LUXEMBOURG TRADITIONAL DIAGNOSTIC TEST IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 645 LUXEMBOURG MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 646 LUXEMBOURG MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 647 LUXEMBOURG MOLECULAR DIAGNOSTIC ASSAY IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 648 LUXEMBOURG CARDIOMETABOLIC MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 649 LUXEMBOURG POC ANALYZER IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 650 LUXEMBOURG POC ANALYZER IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 651 LUXEMBOURG CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 652 LUXEMBOURG CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 653 LUXEMBOURG CARDIAC MARKER TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 654 LUXEMBOURG BLOOD GAS/ELECTROLYTE TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 655 LUXEMBOURG BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 656 LUXEMBOURG BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 657 LUXEMBOURG BLOOD GAS/ELECTROLYTE TESTING CONSUMABLES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 658 LUXEMBOURG BLOOD GAS/ELECTROLYTE TESTING INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 659 LUXEMBOURG PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 660 LUXEMBOURG PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 661 LUXEMBOURG PORTABLE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 662 LUXEMBOURG BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 663 LUXEMBOURG BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 664 LUXEMBOURG BENCHTOP IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 665 LUXEMBOURG HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 666 LUXEMBOURG HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 667 LUXEMBOURG HBA1C TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 668 LUXEMBOURG ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 669 LUXEMBOURG ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 670 LUXEMBOURG ECG DEVICE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 671 LUXEMBOURG PREGNANCY AND FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 672 LUXEMBOURG PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 673 LUXEMBOURG PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 674 LUXEMBOURG PREGNANCY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 675 LUXEMBOURG FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 676 LUXEMBOURG FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 677 LUXEMBOURG FERTILITY TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 678 LUXEMBOURG COAGULATION MONITORING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 679 LUXEMBOURG ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 680 LUXEMBOURG ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 681 LUXEMBOURG ANTICOAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 682 LUXEMBOURG VISCOELASTIC COAGULATION MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY METHOD, 2018-2032 (USD THOUSAND)
TABLE 683 LUXEMBOURG DRUG-OF-ABUSE (DOA) TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 684 LUXEMBOURG DOA ANALYZERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 685 LUXEMBOURG DOA ANALYZERS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 686 LUXEMBOURG DOA ANALYZERS MONITORING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 687 LUXEMBOURG RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 688 LUXEMBOURG RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 689 LUXEMBOURG RAPID TESTING DEVICES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 690 LUXEMBOURG URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 691 LUXEMBOURG URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 692 LUXEMBOURG URINALYSIS TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 693 LUXEMBOURG CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 694 LUXEMBOURG CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 695 LUXEMBOURG CHOLESTEROL TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 696 LUXEMBOURG INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 697 LUXEMBOURG INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 698 LUXEMBOURG INSTRUMENTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 699 LUXEMBOURG FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 700 LUXEMBOURG FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 701 LUXEMBOURG FECAL OCCULT TESTING PRODUCTS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 702 LUXEMBOURG POINT-OF-CARE-TESTING (POCT) MARKET, BY PLATFORM, 2018-2032 (USD THOUSAND)
TABLE 703 LUXEMBOURG POINT-OF-CARE-TESTING (POCT) MARKET, BY APPLICATION, 2018-2032 (USD THOUSAND)
TABLE 704 LUXEMBOURG INFECTIOUS DISEASES IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 705 LUXEMBOURG POINT-OF-CARE-TESTING (POCT) MARKET, BY PRESCRIPTION MODE, 2018-2032 (USD THOUSAND)
TABLE 706 LUXEMBOURG POINT-OF-CARE-TESTING (POCT) MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 707 LUXEMBOURG HOSPITALS IN POINT-OF-CARE-TESTING (POCT) MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 708 LUXEMBOURG PRIVATE IN POINT-OF-CARE-TESTING (POCT) MARKET, BY LEVEL, 2018-2032 (USD THOUSAND)
TABLE 709 LUXEMBOURG PUBLIC IN POINT-OF-CARE-TESTING (POCT) MARKET, BY LEVEL, 2018-2032 (USD THOUSAND)
TABLE 710 LUXEMBOURG POINT-OF-CARE-TESTING (POCT) MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
Liste des figures
FIGURE 1 GSA AND BENELUX POINT-OF-CARE-TESTING (POCT) MARKET: SEGMENTATION
FIGURE 2 GSA AND BENELUX POINT-OF-CARE-TESTING (POCT) MARKET: DATA TRIANGULATION
FIGURE 3 GSA AND BENELUX POINT-OF-CARE-TESTING (POCT) MARKET: DROC ANALYSIS
FIGURE 4 GSA POINT-OF-CARE-TESTING (POCT) MARKET: GLOBAL VS REGIONAL MARKET ANALYSIS
FIGURE 5 BENELUX POINT-OF-CARE-TESTING (POCT) MARKET: GLOBAL VS REGIONAL MARKET ANALYSIS
FIGURE 6 GSA AND BENELUX POINT-OF-CARE-TESTING (POCT) MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 7 GSA AND BENELUX POINT-OF-CARE-TESTING (POCT) MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 8 GSA AND BENELUX POINT-OF-CARE-TESTING (POCT) MARKET: DBMR MARKET POSITION GRID
FIGURE 9 GSA AND BENELUX POINT-OF-CARE-TESTING (POCT) MARKET: MARKET END USER COVERAGE GRID
FIGURE 10 GSA AND BENELUX POINT-OF-CARE-TESTING (POCT) MARKET: SEGMENTATION
FIGURE 11 GSA POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE (2024)
FIGURE 12 BEMELUX POINT-OF-CARE-TESTING (POCT) MARKET, BY PRODUCT TYPE (2024)
FIGURE 13 GSA POINT-OF-CARE-TESTING (POCT) MARKET: EXECUTIVE SUMMARY
FIGURE 14 BENELUX POINT-OF-CARE-TESTING (POCT) MARKET: EXECUTIVE SUMMARY
FIGURE 15 STRATEGIC DECISIONS
FIGURE 16 RISING USE OF POC TESTING IN HEALTHCARE FACILITIES IS EXPECTED TO DRIVE THE GSA POINT-OF-CARE-TESTING (POCT) MARKET, IN THE FORECAST PERIOD OF 2025 TO 2032
FIGURE 17 RISING USE OF POC TESTING IN HEALTHCARE FACILITIES IS EXPECTED TO DRIVE THE BENELUX POINT-OF-CARE-TESTING (POCT) MARKET, IN THE FORECAST PERIOD OF 2025 TO 2032
FIGURE 18 GLUCOSE MONITORING PRODUCTS SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE GSA POINT-OF-CARE-TESTING (POCT) MARKET IN THE FORECAST PERIOD OF 2025 & 2032
FIGURE 19 GLUCOSE MONITORING PRODUCTS SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE BENELUX POINT-OF-CARE-TESTING (POCT) MARKET IN THE FORECAST PERIOD OF 2025 & 2032
FIGURE 20 DROC ANALYSIS
FIGURE 21 NUMBER OF PEOPLE USING VARIOUS SUBSTANCES OR EXPERIENCING PROBLEMATIC USE PREVALENCE (PERCENTAGE)
FIGURE 22 NUMBER OF PEOPLE USING VARIOUS SUBSTANCES OR EXPERIENCING PROBLEMATIC USE (IN MILLION)
FIGURE 23 SUBSTANCE USE BEHAVIOURS AMONG 15-YEAR-OLDS IN 2022 (SELF-REPORTED PREVALENCE)
FIGURE 24 GSA POINT-OF-CARE-TESTING (POCT) MARKET: BY PRODUCT TYPE, 2024
FIGURE 25 GSA POINT-OF-CARE-TESTING (POCT) MARKET: BY PRODUCT TYPE, 2025-2032 (USD THOUSAND)
FIGURE 26 GSA POINT-OF-CARE-TESTING (POCT) MARKET: BY PRODUCT TYPE, CAGR (2025-2032)
FIGURE 27 GSA POINT-OF-CARE-TESTING (POCT) MARKET: BY PRODUCT TYPE, LIFELINE CURVE
FIGURE 28 GSA POINT-OF-CARE TESTING (POCT) MARKET: BY PRESCRIPTION MODE, 2024
FIGURE 29 GSA POINT-OF-CARE TESTING (POCT) MARKET: BY PRESCRIPTION MODE, 2025 TO 2032 (USD THOUSAND)
FIGURE 30 GSA POINT-OF-CARE TESTING (POCT) MARKET: BY PRESCRIPTION MODE, CAGR (2025- 2032)
FIGURE 31 GSA POINT-OF-CARE TESTING (POCT) MARKET: BY PRESCRIPTION MODE, LIFELINE CURVE
FIGURE 32 GSA POINT-OF-CARE TESTING (POCT) MARKET: BY PLATFORM, 2024
FIGURE 33 GSA POINT-OF-CARE TESTING (POCT) MARKET: BY PLATFORM, 2025 TO 2032 (USD THOUSAND)
FIGURE 34 GSA POINT-OF-CARE TESTING (POCT) MARKET: BY PLATFORM, CAGR (2025- 2032)
FIGURE 35 GSA POINT-OF-CARE TESTING (POCT) MARKET: BY PLATFORM, LIFELINE CURVE
FIGURE 36 GSA POINT-OF-CARE TESTING (POCT) MARKET: BY APPLICATION, 2024
FIGURE 37 GSA POINT-OF-CARE TESTING (POCT) MARKET: BY APPLICATION, 2025 TO 2032 (USD THOUSAND)
FIGURE 38 GSA POINT-OF-CARE TESTING (POCT) MARKET: BY APPLICATION, CAGR (2025- 2032)..
FIGURE 39 GSA POINT-OF-CARE TESTING (POCT) MARKET: BY APPLICATION, LIFELINE CURVE
FIGURE 40 GSA POINT-OF-CARE TESTING (POCT) MARKET: BY END USER, 2024
FIGURE 41 GSA POINT-OF-CARE TESTING (POCT) MARKET: BY END USER, 2025 TO 2032 (USD THOUSAND)
FIGURE 42 GSA POINT-OF-CARE TESTING (POCT) MARKET: BY END USER, CAGR (2025- 2032)
FIGURE 43 GSA POINT-OF-CARE TESTING (POCT) MARKET: BY END USER, LIFELINE CURVE
FIGURE 44 GSA POINT-OF-CARE TESTING (POCT) MARKET: BY DISTRIBUTION CHANNEL, 2024
FIGURE 45 GSA POINT-OF-CARE TESTING (POCT) MARKET: BY DISTRIBUTION CHANNEL, 2025 TO 2032 (USD THOUSAND)
FIGURE 46 GSA POINT-OF-CARE TESTING (POCT) MARKET: BY DISTRIBUTION CHANNEL, CAGR (2025- 2032)
FIGURE 47 GSA POINT-OF-CARE TESTING (POCT) MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 48 BENELUX POINT-OF-CARE-TESTING (POCT) MARKET: BY PRODUCT TYPE, 2024
FIGURE 49 BENELUX POINT-OF-CARE-TESTING (POCT) MARKET: BY PRODUCT TYPE, 2025-2032 (USD THOUSAND)
FIGURE 50 BENELUX POINT-OF-CARE-TESTING (POCT) MARKET: BY PRODUCT TYPE, CAGR (2025-2032)
FIGURE 51 BENELUX POINT-OF-CARE-TESTING (POCT) MARKET: BY PRODUCT TYPE, LIFELINE CURVE
FIGURE 52 BENELUX POINT-OF-CARE TESTING (POCT) MARKET: BY PLATFORM, 2024
FIGURE 53 BENELUX POINT-OF-CARE TESTING (POCT) MARKET: BY PLATFORM, 2025 TO 2032 (USD THOUSAND)
FIGURE 54 BENELUX POINT-OF-CARE TESTING (POCT) MARKET: BY PLATFORM, CAGR (2025- 2032)
FIGURE 55 BENELUX POINT-OF-CARE TESTING (POCT) MARKET: BY PLATFORM, LIFELINE CURVE
FIGURE 56 BENELUX POINT-OF-CARE TESTING (POCT) MARKET: BY PRESCRIPTION MODE, 2024
FIGURE 57 BENELUX POINT-OF-CARE TESTING (POCT) MARKET: BY PRESCRIPTION MODE, 2025 TO 2032 (USD THOUSAND)
FIGURE 58 BENELUX POINT-OF-CARE TESTING (POCT) MARKET: BY PRESCRIPTION MODE, CAGR (2025- 2032)
FIGURE 59 BENELUX POINT-OF-CARE TESTING (POCT) MARKET: BY PRESCRIPTION MODE, LIFELINE CURVE
FIGURE 60 BENELUX POINT-OF-CARE TESTING (POCT) MARKET: BY APPLICATION, 2024
FIGURE 61 BENELUX POINT-OF-CARE TESTING (POCT) MARKET: BY APPLICATION, 2025 TO 2032 (USD THOUSAND)
FIGURE 62 BENELUX POINT-OF-CARE TESTING (POCT) MARKET: BY APPLICATION, CAGR (2025- 2032)
FIGURE 63 BENELUX POINT-OF-CARE TESTING (POCT) MARKET: BY APPLICATION, LIFELINE CURVE
FIGURE 64 BENELUX POINT-OF-CARE TESTING (POCT) MARKET: BY END USER, 2024
FIGURE 65 BENELUX POINT-OF-CARE TESTING (POCT) MARKET: BY END USER, 2025 TO 2032 (USD THOUSAND)
FIGURE 66 BENELUX POINT-OF-CARE TESTING (POCT) MARKET: BY END USER, CAGR (2025- 2032)
FIGURE 67 BENELUX POINT-OF-CARE TESTING (POCT) MARKET: BY END USER, LIFELINE CURVE
FIGURE 68 BENELUX POINT-OF-CARE TESTING (POCT) MARKET: BY DISTRIBUTION CHANNEL, 2024
FIGURE 69 BENELUX POINT-OF-CARE TESTING (POCT) MARKET: BY DISTRIBUTION CHANNEL, 2025 TO 2032 (USD THOUSAND)
FIGURE 70 BENELUX POINT-OF-CARE TESTING (POCT) MARKET: BY DISTRIBUTION CHANNEL, CAGR (2025- 2032)
FIGURE 71 BENELUX POINT-OF-CARE TESTING (POCT) MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 72 BENELUX POINT-OF-CARE-TESTING (POCT) MARKET: SNAPSHOT (2024)
FIGURE 73 GSA POINT-OF-CARE-TESTING (POCT) MARKET: SNAPSHOT (2024)
FIGURE 74 GSA AND BENELUX POINT-OF-CARE-TESTING (POCT) MARKET: COMPANY SHARE 2024 (%)
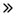
Méthodologie de recherche
La collecte de données et l'analyse de l'année de base sont effectuées à l'aide de modules de collecte de données avec des échantillons de grande taille. L'étape consiste à obtenir des informations sur le marché ou des données connexes via diverses sources et stratégies. Elle comprend l'examen et la planification à l'avance de toutes les données acquises dans le passé. Elle englobe également l'examen des incohérences d'informations observées dans différentes sources d'informations. Les données de marché sont analysées et estimées à l'aide de modèles statistiques et cohérents de marché. De plus, l'analyse des parts de marché et l'analyse des tendances clés sont les principaux facteurs de succès du rapport de marché. Pour en savoir plus, veuillez demander un appel d'analyste ou déposer votre demande.
La méthodologie de recherche clé utilisée par l'équipe de recherche DBMR est la triangulation des données qui implique l'exploration de données, l'analyse de l'impact des variables de données sur le marché et la validation primaire (expert du secteur). Les modèles de données incluent la grille de positionnement des fournisseurs, l'analyse de la chronologie du marché, l'aperçu et le guide du marché, la grille de positionnement des entreprises, l'analyse des brevets, l'analyse des prix, l'analyse des parts de marché des entreprises, les normes de mesure, l'analyse globale par rapport à l'analyse régionale et des parts des fournisseurs. Pour en savoir plus sur la méthodologie de recherche, envoyez une demande pour parler à nos experts du secteur.
Personnalisation disponible
Data Bridge Market Research est un leader de la recherche formative avancée. Nous sommes fiers de fournir à nos clients existants et nouveaux des données et des analyses qui correspondent à leurs objectifs. Le rapport peut être personnalisé pour inclure une analyse des tendances des prix des marques cibles, une compréhension du marché pour d'autres pays (demandez la liste des pays), des données sur les résultats des essais cliniques, une revue de la littérature, une analyse du marché des produits remis à neuf et de la base de produits. L'analyse du marché des concurrents cibles peut être analysée à partir d'une analyse basée sur la technologie jusqu'à des stratégies de portefeuille de marché. Nous pouvons ajouter autant de concurrents que vous le souhaitez, dans le format et le style de données que vous recherchez. Notre équipe d'analystes peut également vous fournir des données sous forme de fichiers Excel bruts, de tableaux croisés dynamiques (Fact book) ou peut vous aider à créer des présentations à partir des ensembles de données disponibles dans le rapport.