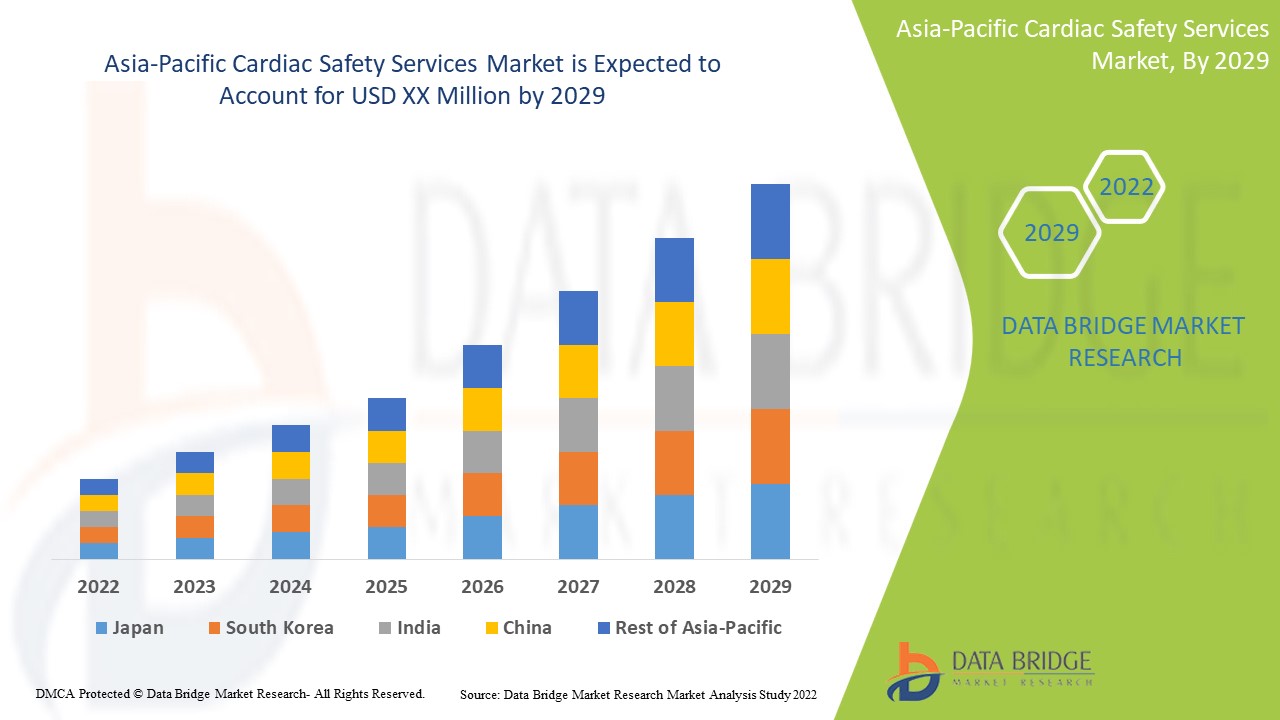
アジア太平洋の心臓安全サービス市場、サービス別(ECG/ホルター測定、血圧測定、体外心臓安全評価サービス、心血管イメージング、リアルタイム遠隔測定モニタリング、ECGSの中央オーバーリード、非侵襲性心臓イメージング、生理学的ストレステスト、徹底したQT試験、TQTおよび曝露反応モデリング、血小板凝集およびその他のサービス)、フェーズ別(フェーズ1、フェーズ2、フェーズ3)、タイプ別(統合サービスおよびスタンドアロンサービス)、エンドユーザー別(医薬品およびバイオ医薬品会社、契約研究機関、学術研究機関)業界動向および2029年までの予測。
市場分析と洞察
アジア太平洋の心臓安全サービス市場は、新薬開発の増加、新興企業の増加、技術革新などの要因によって牽引されており、需要が高まり、研究開発への投資も増加しているため、市場の成長につながっています。現在、さまざまな研究調査が行われており、メーカーが新しい革新的な心臓安全サービス システムを開発するための競争上の優位性が生まれ、心臓安全サービス市場にさまざまな機会がもたらされると期待されています。ただし、承認に関する政府の厳しい規制により、成長が妨げられると予想されます。
アジア太平洋の心臓安全サービス市場レポートでは、市場シェア、新開発、製品パイプライン分析、国内および現地の市場プレーヤーの影響の詳細、新たな収益源、市場規制の変更、製品承認、戦略的決定、製品発売、地理的拡張、市場における技術革新の観点からの機会の分析が提供されます。分析と市場シナリオを理解するには、アナリスト概要についてお問い合わせください。当社のチームが、収益に影響を与えるソリューションを作成し、目的の目標を達成するお手伝いをします。さまざまな地域の発展途上国における小売ユニットの拡張性と事業拡大、および機械および医薬品製品の安全な流通のためのサプライヤーとのパートナーシップは、予測期間における市場の需要を推進した主な原動力です。
アジア太平洋地域の心臓安全サービス市場は支援的であり、病気の進行を抑えることを目指しています。データブリッジマーケットリサーチは、アジア太平洋地域の心臓安全サービス市場は2022年から2029年の予測期間中に16.4%のCAGRで成長すると分析しています。
|
レポートメトリック |
詳細 |
|
予測期間 |
2022年から2029年 |
|
基準年 |
2021 |
|
歴史的な年 |
2020 (2019 - 2014 にカスタマイズ可能) |
|
定量単位 |
売上高は百万米ドル、価格は米ドル |
|
対象セグメント |
サービス別 (ECG/ホルター測定、血圧測定、体外心臓安全性評価サービス、心血管画像、リアルタイム遠隔測定モニタリング、ECGS の中央オーバーリード、非侵襲性心臓画像、生理学的ストレステスト、徹底的な QT 試験、TQT および曝露反応モデリング、血小板凝集およびその他のサービス)、フェーズ別 (フェーズ 1、フェーズ 2、フェーズ 3)、タイプ別 (統合サービスおよびスタンドアロン サービス)、エンド ユーザー別 (製薬およびバイオ医薬品会社、契約研究機関、学術研究機関) |
|
対象国 |
中国、日本、韓国、インド、オーストラリア、シンガポール、タイ、マレーシア、インドネシア、フィリピン、その他のアジア太平洋諸国 |
|
対象となる市場プレーヤー |
Koninklijke Philips NV、Laboratory Corporation of America Holdings、IQVIA、Medpace、Ncardia、Certara、Eurofins Scientific、SGS SA、Banook、Celerion、Biotrial、NEXEL Co., Ltd、Richmond Pharmacology、PhysioStim、Shanghai Medicilon Inc、Clario、PPD Inc など。 |
市場定義:
心臓安全サービスは、一般的に、心臓の安全性を監視するために必要な臨床試験やその他の研究のサポートと設計に役立ちます。心臓安全サービス市場の需要は、先進国と発展途上国の両方で増加しており、その理由は臨床試験と製品発売の増加です。心臓安全サービス市場は、革新的な製品の導入、技術製品の増加、可処分所得の増加により成長しています。市場は、新興市場の探索、市場プレーヤーによる戦略的イニシアチブ、医療費の増加により、予測期間中に成長するでしょう。
アジア太平洋地域の心臓安全サービス市場の動向
ドライバー
- 臨床試験数の増加
臨床試験は、数百年前に遡るよく構造化されたシステムであり、現在でも医薬品の承認に必要な規制要件の根幹となっています。最近、臨床試験の分野では大きな進歩があり、臨床試験の数が増加し、市場の成長を促進することが期待されています。
臨床試験の規制にはさまざまな変更があり、臨床試験の数とその肯定的な結果が増加しました。
例えば、
- メディカルニュースの記事によると、全職員の研修義務化など臨床試験の質の向上により、試験数が大幅に増加している。また、2017年にNIHは、NIHが資金提供する試験では、すべての研究者と職員が適正臨床試験基準(GCP)の研修を受ける必要があると述べた。
医療費と資金の増加
国が医療に費やす資金の規模と長期にわたるその成長率は、資金調達の取り決めや医療制度の組織構造など、さまざまな経済的、社会的要因によって左右されます。
Healthcare expenditure has increased across developed countries and emerging economies as the disposable income of people are growing. The more money is spent on healthcare, the healthier a country's population is.
Opportunity
- Increase in new drug development
The clinical trials are vital for discovering and developing new drugs for disease treatment. It is the best way researchers can find out what treatments work or don't work on humans. The drug development is characterized as forming new treatment as medicines or devices for curing various diseases such as cancer, endocrine, metabolic, and others.
- Thus, clinical trials are the most effective way to ensure the safety and efficacy of the therapeutic drug before launching in the market and human consumption that includes cardiac safety evaluation which is an essential part before any medical product goes into the market
Restraint/Challenge
The proper assessment and reporting of clinical cardiac safety data is essential. Approval and product recall for any medical products depend on cardiac safety evaluation. So it is necessary to provide and conduct cardiac safety evaluation according to the legal procedure, otherwise it leads to late approval of the product which is expected to restrain the market growth.
For instance,
- According to the article by IQVIA, there were 47 instances of post-marketing withdrawal of drugs between 1957 and 2007, in which 45% of these were due to concerns regarding cardiovascular toxicity. Similarly, 27% of the potential new drug molecules that failed in the preclinical phase in the last two decades did so because of cardiovascular toxicity as they did not meet the required regulatory
Asia-Pacific Cardiac Safety Services Market Segmentation
Asia-Pacific cardiac safety services market is categorized into type, services, phase and end user. The growth among segments helps you analyse niche pockets of growth and strategies to approach the market and determine your core application areas and the difference in your target markets.
Services
- ECG/holter measurements
- Blood pressure measurements
- In vitro cardiac safety assessment services
- Cardiovascular imaging
- Real-time telemetry monitoring
- Central over-read of ECGS
- Non-invasive cardiac imaging
- Physiologic stress testing
- Thorough QT studies
- TQT and exposure response modeling
- Platelet aggregation
- Other services
On the basis of services, the cardiac safety services market is segmented into ECG/Holter measurements, blood pressure measurements, in vitro cardiac safety assessment services, cardiovascular imaging, real-time telemetry monitoring, central over-read of ECGS, non-invasive cardiac imaging, physiologic stress testing, thorough QT studies, TQT and exposure response modeling, platelet aggregation and other services
Phase
- Phase 1
- Phase 2
- Phase 3
On the basis of phases, the cardiac safety services market is segmented into phase 1, phase 2, and phase 3.
Type
- Integrated services
- Standalone services
On the basis of type, the cardiac safety services market is segmented into integrated services and standalone services.
End User
- Pharmaceuticals & Biopharmaceuticals Companies
- Contract Research Organizations
- Academic and Research Institute
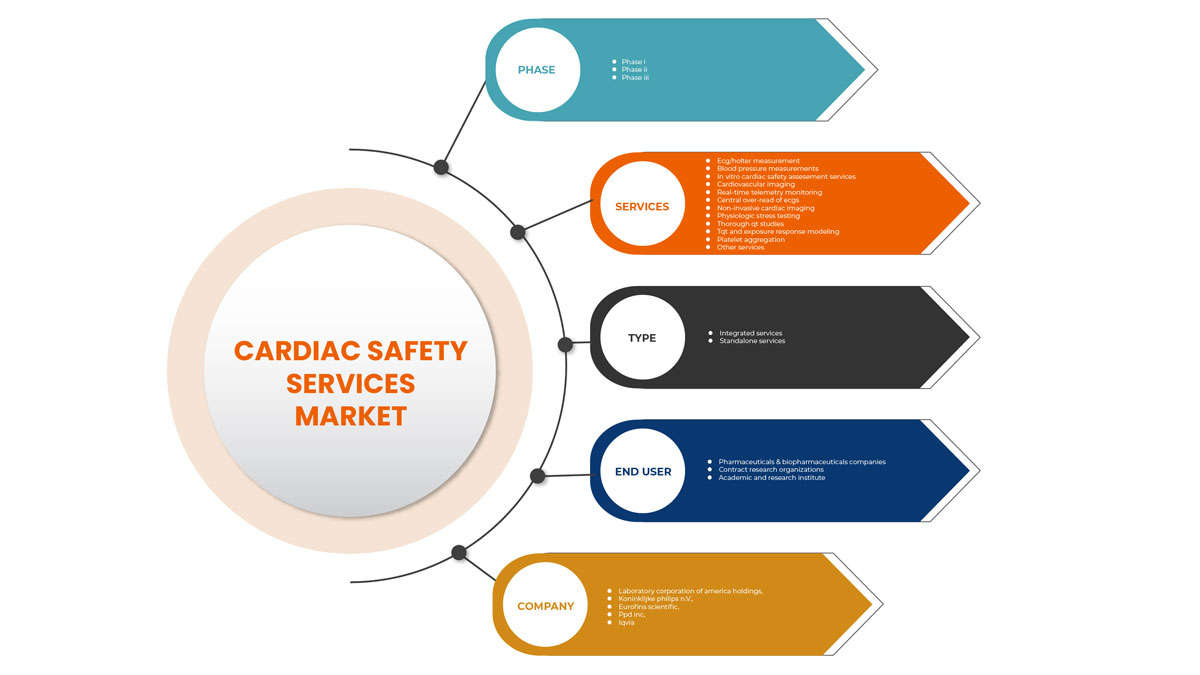
On the basis of end user, the cardiac safety services market is segmented pharmaceuticals & biopharmaceuticals companies, contract research organizations, and academic and research institute.
Cardiac Safety Services Regional Analysis/Insights
The cardiac safety services is analysed and market size insights and trends are provided by type, services, phase and end user as referenced above.
The countries covered in the cardiac safety services report are China, Japan, South Korea, India, Australia, Singapore, Thailand, Malaysia, Indonesia, Philippines and Rest of Asia-Pacific.
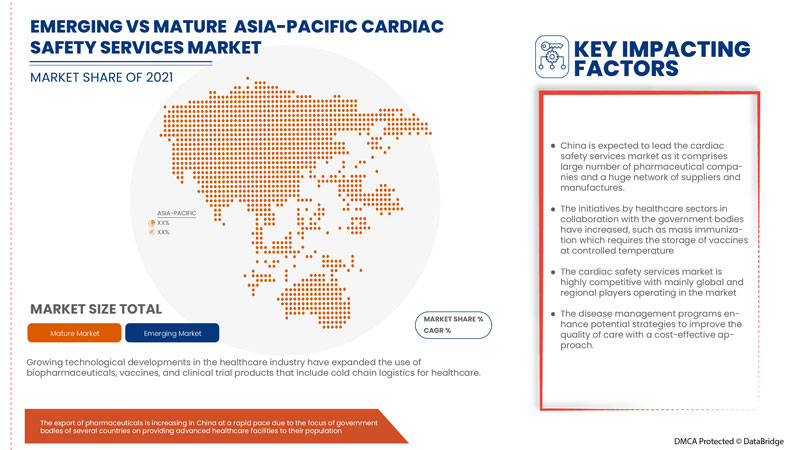
China is expected to dominate due to increase in number of strategic action taken by major market players.
The country section of the report also provides individual market impacting factors and changes in market regulation that impact the current and future trends of the market. Data points such as down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Competitive Landscape and Cardiac Safety Services Analysis
Asia-Pacific cardiac safety services market competitive landscape provides details by the competitors. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, Asia-Pacific presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, and application dominance. The above data points provided are only related to the companies' focus on cardiac safety services market.
Some of the major players are Koninklijke Philips N.V., Laboratory Corporation of America Holdings, IQVIA, Medpace, Ncardia, Certara, Eurofins Scientific, SGS SA, Banook, and Celerion among others.
Research Methodology
データ収集と基準年分析は、大規模なサンプル サイズのデータ収集モジュールを使用して行われます。市場データは、市場統計モデルとコヒーレント モデルを使用して分析および推定されます。さらに、市場シェア分析と主要トレンド分析は、市場レポートの主要な成功要因です。DBMR 調査チームが使用する主要な調査方法は、データ マイニング、データ変数の市場への影響の分析、および一次 (業界の専門家) 検証を含むデータ三角測量です。これとは別に、データ モデルには、ベンダー ポジショニング グリッド、市場タイムライン分析、市場概要とガイド、企業ポジショニング グリッド、企業市場シェア分析、測定基準、アジア太平洋と地域、ベンダー シェア分析が含まれます。さらに質問がある場合は、アナリストへの電話をリクエストしてください。
SKU-
世界初のマーケットインテリジェンスクラウドに関するレポートにオンラインでアクセスする
- インタラクティブなデータ分析ダッシュボード
- 成長の可能性が高い機会のための企業分析ダッシュボード
- カスタマイズとクエリのためのリサーチアナリストアクセス
- インタラクティブなダッシュボードによる競合分析
- 最新ニュース、更新情報、トレンド分析
- 包括的な競合追跡のためのベンチマーク分析のパワーを活用
目次
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 SERVICES LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET END USER GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PORTERS FIVE FORCES
4.2 PESTEL ANALYSIS
5 EPIDEMIOLOGY
5.1 INCIDENCE OF ALL BY GENDER
5.2 TREATMENT RATE
5.3 MORTALITY RATE
5.4 DRUG ADHERENCE AND THERAPY SWITCH MODEL
6 INDUSTRY INSIGHT
6.1 DEMOGRAPHIC TRENDS: IMPACTS ON ALL INCIDENCE RATES
6.2 PATENT ANALYSIS
6.3 PATENT FLOW DIAGRAM
6.4 KEY PATIENT ENROLLMENT STRATEGIES
6.5 PRICING STRATEGY
7 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET: REGULATIONS
8 MARKET OVERVIEW
8.1 DRIVERS
8.1.1 INCREASE IN THE NUMBER OF CLINICAL TRIALS
8.1.2 INCREASE IN HEALTHCARE EXPENDITURE AND FUNDING
8.1.3 INCREASE IN STRATEGIC INITIATIVES BY MAJOR MARKET PLAYERS
8.1.4 INCREASE IN R&D ACTIVITIES
8.2 RESTRAINTS
8.2.1 HIGH COST OF CARDIAC SAFETY EVALUATION
8.2.2 STRICT REGULATORY
8.3 OPPORTUNITIES:
8.3.1 INCREASE IN NEW DRUG DEVELOPMENT
8.3.2 RISE IN THE EXPANSION OF THE CARDIAC SAFETY SERVICES
8.4 CHALLENGES
8.4.1 TIME-CONSUMING PROCEDURE
8.4.2 LACK OF SKILLED PERSON TO OPERATE DEVICES DURING CARDIAC SAFETY EVALUATION
9 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET, BY SERVICES
9.1 OVERVIEW
9.2 ECG/HOLTER MEASUREMENTS
9.3 BLOOD PRESSURE MEASUREMENTS
9.4 IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES
9.4.1 HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS
9.4.1.1 1 CONCENTRATIONS
9.4.1.2 4 CONCENTRATIONS
9.4.2 COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA)
9.4.2.1 3 CONCENTRATIONS
9.4.2.2 5 CONCENTRATIONS
9.4.3 IN VITRO HERG ASSAY
9.4.4 OTHERS
9.5 CARDIOVASCULAR IMAGING
9.6 REAL TIME TELEMETRY MONITORING
9.7 CENTRAL OVER-READ OF ECGS
9.8 NON-INVASIVE CARDIAC IMAGING
9.9 PHYSIOLOGIC STRESS TESTING
9.1 THOROUGH QT STUDIES
9.11 TQT AND EXPOSURE RESPONSE MODELLING
9.12 PLATELET AGGREGATION
9.13 OTHERS
10 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET, BY PHASE
10.1 OVERVIEW
10.2 PHASE I
10.3 PHASE II
10.4 PHASE III
11 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET, BY TYPE
11.1 OVERVIEW
11.2 INTEGRATED SERVICES
11.3 STANDALONE SERVICES
12 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET, BY END USER
12.1 OVERVIEW
12.2 PHARMACEUTICALS & BIOPHARMACEUTICALS COMPANIES
12.3 CONTRACT RESEARCH ORGANIZATIONS
12.4 ACADEMIC AND RESEARCH INSTITUTE
13 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET, BY REGION
13.1 ASIA-PACIFIC
13.1.1 CHINA
13.1.2 JAPAN
13.1.3 SOUTH KOREA
13.1.4 INDIA
13.1.5 AUSTRALIA
13.1.6 SINGAPORE
13.1.7 THAILAND
13.1.8 MALAYSIA
13.1.9 INDONESIA
13.1.10 PHILIPPINES
13.1.11 REST OF ASIA-PACIFIC
14 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET: COMPANY LANDSCAPE
14.1 COMPANY SHARE ANALYSIS: ASIA PACIFIC
15 COMPANY PROFILE
15.1 EUROFINS SCIENTIFIC
15.1.1 COMPANY SNAPSHOT
15.1.2 REVENUE ANALYSIS
15.1.3 COMPANY SHARE ANALYSIS
15.1.4 PRODUCT PORTFOLIO
15.1.5 RECENT DEVELOPMENTS
15.1.5.1 AGREEMENTS
15.1.5.2 ACQUISITION
15.2 PPD INC. (SUBSIDIARY OF THERMO FISHER SCIENTIFIC INC)
15.2.1 COMPANY SNAPSHOT
15.2.2 REVENUE ANALYSIS
15.2.3 COMPANY SHARE ANALYSIS
15.2.4 PRODUCT PORTFOLIO
15.2.5 RECENT DEVELOPMENT
15.2.5.1 INVESTMENT
15.3 KONINKLIJKE PHILIPS N.V.
15.3.1 COMPANY SNAPSHOT
15.3.2 REVENUE ANALYSIS
15.3.3 COMPANY SHARE ANALYSIS
15.3.4 PRODUCT PORTFOLIO
15.3.5 RECENT DEVELOPMENT
15.3.5.1 ACQUISITION
15.4 IQVIA
15.4.1 COMPANY SNAPSHOT
15.4.2 REVENUE ANALYSIS
15.4.3 COMPANY SHARE ANALYSIS
15.4.4 PRODUCT PORTFOLIO
15.4.5 RECENT DEVELOPMENTS
15.4.5.1 ACQUISITION
15.5 LABORATORY CORPORATION OF AMERICA HOLDINGS
15.5.1 COMPANY SNAPSHOT
15.5.2 REVENUE ANALYSIS
15.5.3 COMPANY SHARE ANALYSIS
15.5.4 PRODUCT PORTFOLIO
15.5.5 RECENT DEVELOPMENTS
15.5.5.1 NEW LABORATORY
15.5.5.2 ACQUISITION
15.6 BANOOK
15.6.1 COMPANY SNAPSHOT
15.6.2 PRODUCT PORTFOLIO
15.6.3 RECENT DEVELOPMENT
15.6.3.1 AGREEMENT
15.7 BIOTRIAL
15.7.1 COMPANY SNAPSHOT
15.7.2 PRODUCT PORTFOLIO
15.7.3 RECENT DEVELOPMENT
15.7.3.1 NEW CENTER OPENING
15.8 CELERION
15.8.1 COMPANY SNAPSHOT
15.8.2 PRODUCT PORTFOLIO
15.8.3 RECENT DEVELOPMENT
15.8.3.1 NEW CENTER OPENING
15.9 CERTARA
15.9.1 COMPANY SNAPSHOT
15.9.2 REVENUE ANALYSIS
15.9.3 PRODUCT PORTFOLIO
15.9.4 RECENT DEVELOPMENTS
15.9.4.1 CONTRACT
15.9.4.2 ACQUISITION
15.1 CLARIO
15.10.1 COMPANY SNAPSHOT
15.10.2 PRODUCT PORTFOLIO
15.10.3 RECENT DEVELOPMENT
15.10.3.1 PRODUCT EXPANSION
15.11 MEDPACE
15.11.1 COMPANY SNAPSHOT
15.11.2 REVENUE ANALYSIS
15.11.3 PRODUCT PORTFOLIO
15.11.4 RECENT DEVELOPMENTS
15.11.4.1 ACQUISITION
15.12 NCARDIA
15.12.1 COMPANY SNAPSHOT
15.12.2 PRODUCT PORTFOLIO
15.12.3 RECENT DEVELOPMENT
15.12.3.1 PARTNERSHIP
15.13 NEXEL CO., LTD
15.13.1 COMPANY SNAPSHOT
15.13.2 PRODUCT PORTFOLIO
15.13.3 RECENT DEVELOPMENTS
15.13.3.1 JOINT VENTURE
15.13.3.2 PARTNERSHIP
15.14 PHYSIOSTIM
15.14.1 COMPANY SNAPSHOT
15.14.2 PRODUCT PORTFOLIO
15.14.3 RECENT DEVELOPMENT
15.14.3.1 PARTNERSHIP
15.15 RICHMOND PHARMACOLOGY
15.15.1 COMPANY SNAPSHOT
15.15.2 PRODUCT PORTFOLIO
15.15.3 RECENT DEVELOPMENT
15.15.3.1 EVENT
15.16 SGS SA
15.16.1 COMPANY SNAPSHOT
15.16.2 REVENUE ANALYSIS
15.16.3 PRODUCT PORTFOLIO
15.16.4 RECENT DEVELOPMENT
15.16.4.1 ACQUISITION
15.17 SHANGHAI MEDICILON INC.
15.17.1 COMPANY SNAPSHOT
15.17.2 PRODUCT PORTFOLIO
15.17.3 RECENT DEVELOPMENTS
15.17.3.1 PARTNERSHIP
15.17.3.2 PARTNERSHIP
16 QUESTIONNAIRE
17 RELATED REPORTS
表のリスト
TABLE 1 PROPORTION OF WOMEN IN CLINICAL STUDIES, ACCORDING TO DEVELOPMENT PHASE
TABLE 2 PROBABILITY OF SUCCESS BY CLINICAL TRIAL PHASE TO THERAPEUTIC AREA
TABLE 3 MORTALITY RATES FROM CLINICAL TRIALS AND EUROPEAN SAFETY AND EXPOSURE SURVEY (ESES), DEATHS PER 100 (PYE)
TABLE 4 ADHERENCE RATE TO COMMON CARDIOVASCULAR MEDICATION
TABLE 5 PROPORTION OF WOMEN IN CLINICAL STUDIES, ACCORDING TO DEVELOPMENT PHASE
TABLE 6 INITIATIVES TO INCREASE ENROLLMENT IN CLINICAL TRIALS AMONG UNDERREPRESENTED POPULATIONS
TABLE 7 ESTIMATED COST OF CARDIAC SAFETY EVALUATION DEVICES
TABLE 8 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 9 ASIA PACIFIC ECG/HOLTER MEASUREMENTS IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 10 ASIA PACIFIC BLOOD PRESSURE MEASUREMENTS IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 11 ASIA PACIFIC IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 12 ASIA PACIFIC IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 13 ASIA PACIFIC HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 14 ASIA PACIFIC COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 15 ASIA PACIFIC CARDIOVASCULAR IMAGING IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 16 ASIA PACIFIC REAL TIME TELEMETRY MONITORING IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 17 ASIA PACIFIC CENTRAL OVER-READ OF ECGS IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 18 ASIA PACIFIC NON-INVASIVE CARDIAC IMAGING IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 19 ASIA PACIFIC PHYSIOLOGIC STRESS TESTING IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 20 ASIA PACIFIC THOROUGH QT STUDIES IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 21 ASIA PACIFIC TQT AND EXPOSURE RESPONSE MODELLING IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 22 ASIA PACIFIC PLATELET AGGREGATION IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 23 ASIA PACIFIC OTHERS IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 24 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 25 ASIA PACIFIC PHASE I IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 26 ASIA PACIFIC PHASE II IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 27 ASIA PACIFIC PHASE III IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 28 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 29 ASIA PACIFIC INTEGRATED SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 30 ASIA PACIFIC STANDALONE SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 31 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 32 ASIA PACIFIC PHARMACEUTICALS & BIOPHARMACEUTICALS COMPANIES IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 33 ASIA PACIFIC CONTRACT RESEARCH ORGANIZATIONS IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 34 ASIA PACIFIC ACADEMIC AND RESEARCH INSTITUTE IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 35 ASIA-PACIFIC CARDIAC SAFETY SERVICES MARKET, BY COUNTRY, 2020-2029 (USD MILLION)
TABLE 36 ASIA-PACIFIC CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 37 ASIA-PACIFIC IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 38 ASIA-PACIFIC HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 39 ASIA-PACIFIC COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 40 ASIA-PACIFIC CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 41 ASIA-PACIFIC CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 42 ASIA-PACIFIC CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 43 CHINA CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 44 CHINA IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 45 CHINA HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 46 CHINA COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 47 CHINA CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 48 CHINA CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 49 CHINA CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 50 JAPAN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 51 JAPAN IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 52 JAPAN HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 53 JAPAN COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 54 JAPAN CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 55 JAPAN CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 56 JAPAN CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 57 SOUTH KOREA CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 58 SOUTH KOREA IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 59 SOUTH KOREA HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 60 SOUTH KOREA COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 61 SOUTH KOREA CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 62 SOUTH KOREA CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 63 SOUTH KOREA CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 64 INDIA CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 65 INDIA IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 66 INDIA HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 67 INDIA COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 68 INDIA CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 69 INDIA CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 70 INDIA CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 71 AUSTRALIA CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 72 AUSTRALIA IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 73 AUSTRALIA HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 74 AUSTRALIA COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 75 AUSTRALIA CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 76 AUSTRALIA CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 77 AUSTRALIA CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 78 SINGAPORE CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 79 SINGAPORE IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 80 SINGAPORE HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 81 SINGAPORE COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 82 SINGAPORE CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 83 SINGAPORE CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 84 SINGAPORE CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 85 THAILAND CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 86 THAILAND IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 87 THAILAND HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 88 THAILAND COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 89 THAILAND CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 90 THAILAND CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 91 THAILAND CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 92 MALAYSIA CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 93 MALAYSIA IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 94 MALAYSIA HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 95 MALAYSIA COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 96 MALAYSIA CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 97 MALAYSIA CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 98 MALAYSIA CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 99 INDONESIA CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 100 INDONESIA IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 101 INDONESIA HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 102 INDONESIA COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 103 INDONESIA CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 104 INDONESIA CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 105 INDONESIA CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 106 PHILIPPINES CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 107 PHILIPPINES IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 108 PHILIPPINES HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 109 PHILIPPINES COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 110 PHILIPPINES CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 111 PHILIPPINES CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 112 PHILIPPINES CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 113 REST OF ASIA-PACIFIC CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
図表一覧
FIGURE 1 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET: SEGMENTATION
FIGURE 2 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET: DATA TRIANGULATION
FIGURE 3 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET: DROC ANALYSIS
FIGURE 4 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET: ASIA PACIFIC VS COUNTRY MARKET ANALYSIS
FIGURE 5 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET: DBMR MARKET POSITION GRID
FIGURE 8 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET: MARKET END USER GRID
FIGURE 9 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET: SEGMENTATION
FIGURE 11 THE INCREASE IN DEMAND FOR CARDIAC SAFETY SERVICES ARE EXPECTED TO DRIVE THE ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 12 ECG/HOLTER MEASUREMENTS SUBSTITUTE IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET IN 2022 & 2029
FIGURE 13 PATIENT FLOW DIAGRAM FOR ANY RANDOM DRUG
FIGURE 14 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET
FIGURE 15 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET: BY SERVICES, 2021
FIGURE 16 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET: BY SERVICES, 2022-2029 (USD MILLION)
FIGURE 17 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET: BY SERVICES, CAGR (2022-2029)
FIGURE 18 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET: BY SERVICES, LIFELINE CURVE
FIGURE 19 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET: BY PHASE, 2021
FIGURE 20 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET: BY PHASE, 2022-2029 (USD MILLION)
FIGURE 21 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET: BY PHASE, CAGR (2022-2029)
FIGURE 22 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET: BY PHASE, LIFELINE CURVE
FIGURE 23 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET: BY TYPE, 2021
FIGURE 24 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET: BY TYPE, 2022-2029 (USD MILLION)
FIGURE 25 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET: BY TYPE, CAGR (2022-2029)
FIGURE 26 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET: BY TYPE, LIFELINE CURVE
FIGURE 27 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET: BY END USER, 2021
FIGURE 28 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET: BY END USER, 2022-2029 (USD MILLION)
FIGURE 29 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET: BY END USER, CAGR (2022-2029)
FIGURE 30 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET: BY END USER, LIFELINE CURVE
FIGURE 31 ASIA-PACIFIC CARDIAC SAFETY SERVICES MARKET: SNAPSHOT (2021)
FIGURE 32 ASIA-PACIFIC CARDIAC SAFETY SERVICES MARKET: BY COUNTRY (2021)
FIGURE 33 ASIA-PACIFIC CARDIAC SAFETY SERVICES MARKET: BY COUNTRY (2022 & 2029)
FIGURE 34 ASIA-PACIFIC CARDIAC SAFETY SERVICES MARKET: BY COUNTRY (2021 & 2029)
FIGURE 35 ASIA-PACIFIC CARDIAC SAFETY SERVICES MARKET: BY SERVICES (2022-2029)
FIGURE 36 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET: COMPANY SHARE 2021 (%)
調査方法
データ収集と基準年分析は、大規模なサンプル サイズのデータ収集モジュールを使用して行われます。この段階では、さまざまなソースと戦略を通じて市場情報または関連データを取得します。過去に取得したすべてのデータを事前に調査および計画することも含まれます。また、さまざまな情報ソース間で見られる情報の不一致の調査も含まれます。市場データは、市場統計モデルと一貫性モデルを使用して分析および推定されます。また、市場シェア分析と主要トレンド分析は、市場レポートの主要な成功要因です。詳細については、アナリストへの電話をリクエストするか、お問い合わせをドロップダウンしてください。
DBMR 調査チームが使用する主要な調査方法は、データ マイニング、データ変数が市場に与える影響の分析、および一次 (業界の専門家) 検証を含むデータ三角測量です。データ モデルには、ベンダー ポジショニング グリッド、市場タイムライン分析、市場概要とガイド、企業ポジショニング グリッド、特許分析、価格分析、企業市場シェア分析、測定基準、グローバルと地域、ベンダー シェア分析が含まれます。調査方法について詳しくは、お問い合わせフォームから当社の業界専門家にご相談ください。
カスタマイズ可能
Data Bridge Market Research は、高度な形成的調査のリーダーです。当社は、既存および新規のお客様に、お客様の目標に合致し、それに適したデータと分析を提供することに誇りを持っています。レポートは、対象ブランドの価格動向分析、追加国の市場理解 (国のリストをお問い合わせください)、臨床試験結果データ、文献レビュー、リファービッシュ市場および製品ベース分析を含めるようにカスタマイズできます。対象競合他社の市場分析は、技術ベースの分析から市場ポートフォリオ戦略まで分析できます。必要な競合他社のデータを、必要な形式とデータ スタイルでいくつでも追加できます。当社のアナリスト チームは、粗い生の Excel ファイル ピボット テーブル (ファクト ブック) でデータを提供したり、レポートで利用可能なデータ セットからプレゼンテーションを作成するお手伝いをしたりすることもできます。