欧州における膝関節炎治療用ヒアルロン酸市場:市場規模、シェア、トレンド分析レポート
Market Size in USD Billion
CAGR :
% 
 USD
646.10 Million
USD
1,186.80 Million
2024
2032
USD
646.10 Million
USD
1,186.80 Million
2024
2032
| 2025 –2032 | |
| USD 646.10 Million | |
| USD 1,186.80 Million | |
|
|
|
|
欧州の膝関節症治療用ヒアルロン酸市場:市場セグメンテーション、製品タイプ別(単回注射(モノファジック)、3回注射レジメン(トリファジック)、5回注射レジメン(ペンタファジック))、処方別(架橋ヒアルロン酸、非架橋ヒアルロン酸)、分子量別(高分子量(HMW)ヒアルロン酸、低分子量(LMW)ヒアルロン酸、中分子量ヒアルロン酸)、治療目標別(粘性補充療法、抗炎症および鎮痛、軟骨保護および再生)、エンドユーザー別(病院、整形外科クリニック、外来手術センター、専門疼痛管理センター)、流通チャネル別(直接入札、小売およびオンライン薬局、その他)、国別(ドイツ、フランス、英国、イタリア、ロシア、スペイン、トルコ、オランダ、スイス、ベルギー)スウェーデン、オーストリア、デンマーク、ノルウェー、ポーランド、その他のヨーロッパ諸国 - 2032年までの業界動向と予測
膝関節炎治療におけるヒアルロン酸市場規模
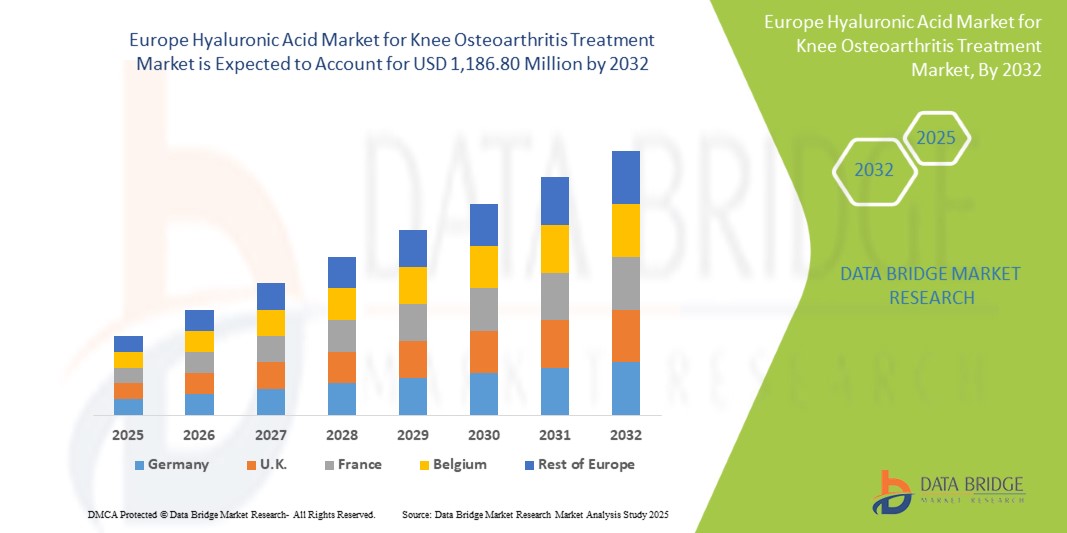
- ヨーロッパの膝関節炎治療用ヒアルロン酸市場は2024年に6億4,610万米ドルと評価され、 2032年までに11億8,680万米ドルに達すると予想されています。
- 2025年から2032年の予測期間中、市場は主に予想される治療法の発売によって7.9%のCAGRで成長する可能性が高い。
- 変形性膝関節症の罹患率の上昇と低侵襲治療への関心の高まりにより、ヒアルロン酸をベースとした治療法の需要が高まっています。さらに、粘性補充療法のメリットに対する認知度の高まりも、市場の成長をさらに促進しています。
膝関節炎治療におけるヒアルロン酸市場分析
- ヒアルロン酸は体内に自然に存在する物質で、特に結合組織、皮膚、関節を潤滑する滑液に多く含まれています。変形性膝関節症の治療において、ヒアルロン酸は粘性補充剤として関節機能の改善と疼痛緩和に用いられます。ヒアルロン酸を膝関節に直接注入することで、クッション効果を高め、運動時の摩擦を軽減し、軟骨の再生を促進することで、変形性関節症の症状に苦しむ患者さんの症状緩和につながります。この治療は、可動域の回復、生活の質の向上、そして病気の進行を遅らせることを目的としています。
- ヨーロッパは、確立された医療システムと、高度な非外科的関節ケアオプションの採用率の高さにより、膝関節炎治療用ヒアルロン酸市場の主要地域として浮上しています。
- 例えば、米国では、患者の意識の高まりと有利な償還政策に支えられ、関節内ヒアルロン酸注射の採用が急増している。
- この地域では、変形性関節症患者の運動機能と生活の質の向上に重点を置いており、ヒアルロン酸ベースの治療法の革新と市場拡大を推進し続けています。
膝関節炎治療におけるヒアルロン酸市場レポートの適用範囲とセグメンテーション
|
属性 |
膝関節炎治療におけるヒアルロン酸市場:主要市場インサイト |
|
対象セグメント |
|
|
対象国 |
|
|
主要な市場プレーヤー |
|
|
市場機会 |
|
|
付加価値データ情報セット |
データブリッジマーケットリサーチがまとめた市場レポートには、市場価値、成長率、セグメンテーション、地理的範囲、主要プレーヤーなどの市場シナリオに関する洞察に加えて、輸出入分析、生産能力の概要、生産消費分析、価格動向分析、気候変動シナリオ、サプライチェーン分析、バリューチェーン分析、原材料/消耗品の概要、ベンダー選択基準、PESTLE分析、ポーター分析、規制枠組みも含まれています。 |
膝関節炎治療におけるヒアルロン酸市場動向
「低侵襲性および標的治療への関心の高まり」
- ヨーロッパの変形性膝関節症治療用ヒアルロン酸市場における重要な傾向として、低侵襲性の標的治療オプションがますます好まれるようになっています。
- ヒアルロン酸注射は粘性補充療法としても知られ、外科的介入を必要とせずに局所的な痛みの緩和と関節可動性の改善をもたらすため、患者と臨床医の両方にとって非常に魅力的です。
- 例えば、 患者のニーズに合わせて調整された単回および複数回の注射製剤が普及し、治療計画に柔軟性と利便性をもたらしている。
- この傾向は、注射技術の進歩、製剤の安定性の向上、治療効果の持続期間の延長によってさらに裏付けられています。
- さらに、超音波ガイド下注射などの画像技術を統合することで、精度と患者の快適性が向上します。
- 非外科的、効果的、そして患者中心の治療オプションへの移行は、変形性膝関節症治療の未来を形作り、ヒアルロン酸市場の成長を促進します。
膝関節炎治療におけるヒアルロン酸市場の動向
ドライバ
「膝関節炎の有病率の増加」
- ヨーロッパでは高齢者人口が増加するにつれ、軟骨の破壊を特徴とする変形性関節症の有病率が著しく増加すると予想されています。この人口動態の傾向は特に憂慮すべきものです。変形性関節症は慢性的な痛み、こわばり、可動性の低下といった症状を引き起こし、患者の生活の質に深刻な影響を与える可能性があるからです。変形性関節症の発症率の上昇に伴い、多くの患者が侵襲的な外科手術に代わる治療法を求めており、効果的な治療法への需要が急増しています。ヒアルロン酸(HA)注射は、患部の関節に潤滑油を供給し、関節機能と可動性を向上させることで症状を効果的に緩和する、低侵襲治療として非常に人気が高まっています。
- これらの注射は、関節液中の天然ヒアルロン酸を補充することで、関節間の摩擦を軽減し、衝撃吸収性を高めることで効果を発揮します。この二重の作用は、痛みを和らげるだけでなく、関節全体の健康状態を改善するため、患者と医療従事者の両方にとって魅力的な選択肢となっています。
例えば、
- 2023年12月、NCBIは、世界中でOAの有病率が高く、高齢者、女性、一部の人種・民族、そして社会経済的地位の低い人々において大きな負担となっていることが研究によって引き続き示されていると述べました。OAの修正可能なリスク要因として最も有力な証拠があるのは、肥満と関節損傷です。
- 2024年11月、Frontiers誌に掲載された記事によると、インドの研究では、年齢層を問わずKOAの有病率が上昇していることが報告されており、50歳未満では19.2%、50~60歳では30.7%、60~70歳では39.7%、70歳以上では54.1%となっている(23)。別の研究では、55歳を過ぎると変形性膝関節症の有病率と発症率の両方が大幅に上昇することが明らかになった。55歳以上の人の平均有病率は13.2%で、男性では9.4%、女性では18.0%となっている。
- The growing awareness of hyaluronic acid's benefits has contributed to its adoption among healthcare professionals and patients alike. As clinical studies and research continue to demonstrate the efficacy of hyaluronic acid in reducing pain and enhancing quality of life for those suffering from knee osteoarthritis, more healthcare providers are incorporating these treatments into their practice. This increasing acceptance, coupled with the rise in osteoarthritis cases, creates a favorable environment for market growth, driving innovation and competition among manufacturers to develop advanced formulations and delivery methods for hyaluronic acid therapies.
Opportunity
“Advancements In Hyaluronic Acid Formulations and Delivery Systems”
- Improved high-molecular-weight and cross-linked hyaluronic acid products provide longer-lasting pain relief and require fewer injections. This appeals to patients seeking effective and convenient solutions for managing their condition. Innovative delivery methods, such as hydrogels and nanoparticle systems, enhance the durability and performance of hyaluronic acid in the joint. These developments meet the rising demand from an aging population. Companies can leverage these technologies to stand out in a competitive market and attract more customers. The focus on reducing treatment frequency while improving patient outcomes strengthens the market’s growth potential. This makes the hyaluronic acid sector for knee osteoarthritis treatment a highly attractive area for investment and expansion
For instance,
- As per IBSA Institut Biochimique SA, Sanofi, Seikagaku Corporation, each company has developed unique formulations to enhance joint lubrication, pain relief, and treatment durability. These innovations focus on improved retention, cross-linking technology, and patient convenience, offering long-lasting symptom relief
- In April 2024 article by sciencedirect highlighted that hyaluronic acid-based liposomes for osteoarthritis drug delivery include surface functionalization after liposome preparation using coupling chemistry or pre-synthesized hyaluronic acid-lipid conjugates through reductive amination. These methods improve biocompatibility, enable simultaneous delivery of various drugs, and enhance control over conjugation, optimizing liposomal systems for effective arthritis treatment
- Advancements in hyaluronic acid formulations and delivery systems significantly enhance the effectiveness and convenience of knee osteoarthritis treatment. By improving product longevity and reducing the frequency of injections, these innovations cater to the growing demand for minimally invasive solutions among an aging population. Companies that embrace these technologies can strengthen their market presence, attract more patients, and capitalize on industry growth. This makes investment in hyaluronic acid-based treatments a strategic opportunity for long-term success in the evolving healthcare landscape.
Restraint/Challenge
“Cost-Related Challenges and Accessibility Concerns For Hyaluronic Acid Therapy In Knee Osteoarthritis”
- Hyaluronic acid (HA) injections for knee osteoarthritis can be prohibitively expensive, particularly when insurance plans either do not cover them fully or exclude them entirely from their benefits. This financial burden often forces patients to pay out-of-pocket, which can be a significant challenge, especially for those with limited financial resources.
- Moreover, multiple injections may be required over time, increasing the cost of treatment. As a result, patients may delay or forgo HA therapy altogether, despite its potential to alleviate pain and improve joint function. The high cost of treatment and lack of insurance coverage often limit accessibility, creating disparities in care. Addressing these financial challenges is vital for making HA therapy more accessible to a wider range of patients, ensuring that more people can benefit from this effective treatment for knee osteoarthritis.
For instance,
- In February 2024, Findings from Pain Physician indicate that Patients receiving HA injections faced significantly higher median costs, especially with multiple injections, compared to those receiving corticosteroid (CS) injections or no injections. Additionally, those requiring total knee arthroplasty (TKA) had higher costs with HA treatment, highlighting the financial barriers associated with this therapy
- As per Sanofi, Enovis and biovico , the high cost of hyaluronic acid (HA) therapy for knee osteoarthritis, such as Synvisc One (USD 232.81) and Biolevox Ha (USD 323.53), limits accessibility. Many patients struggle with affordability, especially without insurance. Lower-cost options, better coverage, and pricing reforms are essential for improving access to these treatments
- The high expense of hyaluronic acid (HA) injections and limited insurance coverage create substantial barriers for many knee osteoarthritis patients. These financial challenges, including the need for multiple injections, often lead patients to delay or abandon HA therapy despite its proven benefits in pain relief and joint function improvement. To improve accessibility and ensure that more individuals can benefit from this treatment, it is essential to address these cost-related issues. Expanding insurance coverage and reducing out-of-pocket expenses will help make HA therapy more accessible, ultimately leading to better care for those suffering from knee osteoarthritis.
Hyaluronic Acid Market for Knee Osteoarthritis Treatment Market Scope
The market is segmented on the basis type, product type, absorption site, age group, source, delivery method, gender, and distribution channel.
|
Segmentation |
Sub-Segmentation |
|
By Product Type |
|
|
By Formulation |
|
|
By Molecular Weight |
|
|
治療目標別
|
|
|
エンドユーザー別 |
|
|
流通チャネル別 |
|
膝関節炎治療におけるヒアルロン酸市場:地域分析
「膝関節炎治療用ヒアルロン酸市場において、ドイツは主要国である」
- ドイツは、先進的な医療インフラ、非外科的関節療法の早期導入、そして主要な市場プレーヤーの強力な存在に支えられ、膝関節炎治療用のヒアルロン酸市場をリードしています。
- ドイツは、変形性膝関節症の発症率の上昇、高齢化人口の増加、関節内ヒアルロン酸注射などの低侵襲治療の選好の増加により、大きな市場シェアを占めています。
- 有利な償還ポリシー、確立された規制枠組み、医療費の増加は、この地域の市場の成長にさらに貢献しています。
- さらに、患者の可動性と生活の質の向上に重点を置き、HA製剤と投与技術の革新と相まって、ヨーロッパ全域で市場拡大を推進し続けています。
「フランスは最高の成長率を記録すると予測されている」
- フランス地域は、急速に進化する医療インフラ、関節の健康に対する意識の高まり、低侵襲治療オプションの需要の増加により、膝関節炎治療用のヒアルロン酸市場において最も急速な成長が見込まれています。
- ドイツ、フランス、英国などの国は、高齢者人口の増加と変形性膝関節症の罹患率の上昇により、重要な市場として浮上しています。
- フランスは、確立された医療システムと高齢者介護の高水準を備え、高度な HA ベースの粘性補充療法の導入をリードしています。
- フランスと英国では、患者数が多く、医療投資が増加し、非外科的変形性関節症治療の受け入れが増えていることが、市場拡大の原動力となっています。
- 国内外のHAメーカーの存在と、整形外科治療へのアクセス改善に向けた政府の取り組みにより、地域全体の市場成長がさらに加速しています。
膝関節炎治療におけるヒアルロン酸市場シェア
市場競争環境は、競合他社ごとに詳細な情報を提供します。企業概要、財務状況、収益、市場ポテンシャル、研究開発投資、新規市場への取り組み、欧州におけるプレゼンス、生産拠点・設備、生産能力、強みと弱み、製品投入、製品群の幅広さ、アプリケーションにおける優位性などの詳細が含まれます。上記のデータは、各社の市場への注力分野にのみ関連しています。
市場で活動している主要なマーケットリーダーは次のとおりです。
- LG化学(韓国)
- バイオベンタスLLC(米国)
- Seikagaku Corporation (Japan)
- サノフィ(フランス)
- アニカ・セラピューティクス社(米国)
- IBSA Biochemical Institute SA (スイス)
- ビアトリス(米国)
- ジマー・バイオメット(米国)
- フィディア・ファーマセウチSpA(イタリア)
- フェリング・ファーマシューティカルズ(スイス)
- TRB CHEMEDICA SA(スイス)
- 杭州星清医療製品有限公司(中国)
- ハンミ製薬株式会社(韓国)
- ヴィルショウ・バイオテック(インド)
- ユープラクシア・ファーマシューティカルズ(カナダ)
欧州における膝関節炎治療用ヒアルロン酸市場の最新動向
- 2024年7月、LG化学は中国の変形性関節症治療市場に参入しました。同社は易帆製薬と提携し、変形性関節症治療薬シノビアン注射剤を中国で発売しました。これにより、同社はポートフォリオの拡大と市場参入を果たしました。
- ユープラクシア・ファーマシューティカルズは2023年5月、変形性関節症(OA)治療薬EP-104IARの第2相臨床試験における最後の患者訪問を完了したと発表しました。同社は第2四半期にトップラインデータを発表する予定で、有意な疼痛緩和と患者機能の改善、そして有望な安全性プロファイルを示すことが期待されています。
- IBSAは2025年3月、販売代理店のLunatusと提携し、サウジアラビアとUAEにおける変形性関節症治療の拡大を目指します。この戦略的提携は、疼痛管理ソリューションの強化を目的としており、変形性関節症患者における高度な治療への高まる需要に対応するため、他の地域諸国への展開も検討しています。
- ユープラクシア・ファーマシューティカルズは2023年6月、米国食品医薬品局(FDA)が膝関節炎(OA)治療薬EP-104IARにファストトラック指定を付与したことを発表しました。この指定は、開発と規制当局の審査を迅速化し、FDAとのやり取りを迅速化することを目指しています。同社は第2相試験を進めており、トップラインデータは2023年第2四半期に得られる見込みです。
SKU-
世界初のマーケットインテリジェンスクラウドに関するレポートにオンラインでアクセスする
- インタラクティブなデータ分析ダッシュボード
- 成長の可能性が高い機会のための企業分析ダッシュボード
- カスタマイズとクエリのためのリサーチアナリストアクセス
- インタラクティブなダッシュボードによる競合分析
- 最新ニュース、更新情報、トレンド分析
- 包括的な競合追跡のためのベンチマーク分析のパワーを活用
目次
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF THE EUROPE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT
1.4 CURRENCY AND PRICING
1.5 LIMITATIONS
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 DBMR TRIPOD DATA VALIDATION MODEL
2.5 MULTIVARIATE MODELLING
2.6 TREATMENT TYPE LIFELINE CURVE
2.7 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.8 DBMR MARKET POSITION GRID
2.9 MARKET END USER COVERAGE GRID
2.1 VENDOR SHARE ANALYSIS
2.11 SECONDARY SOURCES
2.12 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTAL ANALYSIS
4.2 PORTERS FIVE FORCES ANALYSIS
4.3 AVERAGE SELLING PRICE (ASP) ANALYSIS
4.4 MICRO AND MACRO ECONOMIC FACTORS
4.5 KEY PRICING STRATEGIES
4.6 HEALTHCARE ECONOMY
4.7 PENETRATION AND GROWTH PROSPECT MAPPING
4.8 TECHNOLOGY ROADMAP
4.9 VALUE CHAIN ANALYSIS
5 COMPANY-WISE OVERVIEW OF INTRA-ARTICULAR HYALURONIC ACID (HA) INJECTION PRODUCTS BASED ON MOLECULAR WEIGHT -
6 EUROPE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, REGULATORY FRAMEWORK
6.1 NORTH AMERICA
6.2 EUROPE
6.3 ASIA-PACIFIC
6.4 MIDDLE EAST AND AFRICA
7 MARKET OVERVIEW
7.1 DRIVERS
7.1.1 INCREASING PREVALENCE OF KNEE OSTEOARTHRITIS
7.1.2 GROWING PREFERENCE FOR MINIMALLY INVASIVE AND NON-SURGICAL TREATMENT OPTIONS
7.1.3 CLINICAL VALIDATION OF HYALURONIC ACID IN OSTEOARTHRITIS TREATMENT
7.1.4 RECENT LAUNCHES OF HYALURONIC ACID FOR OSTEOARTHRITIS TREATMENT
7.2 RESTRAINTS
7.2.1 COMPETITION OF ALTERNATIVE THERAPIES FOR KNEE OSTEOARTHRITIS
7.2.2 REGULATORY CHALLENGES FACED BY THE MANUFACTURERS IN THE HYALURONIC ACID MARKET
7.3 OPPORTUNITIES
7.3.1 ADVANCEMENTS IN HYALURONIC ACID FORMULATIONS AND DELIVERY SYSTEMS
7.3.2 RAISING AWARENESS AND ACCEPTANCE OF HYALURONIC ACID FOR KNEE OSTEOARTHRITIS TREATMENT AMONG PHYSICIANS AND PATIENTS
7.3.3 THE IMPACT OF COMBINING HYALURONIC ACID WITH OTHER THERAPEUTIC OPTIONS FOR KNEE OSTEOARTHRITIS
7.4 CHALLENGES
7.4.1 COST-RELATED CHALLENGES AND ACCESSIBILITY CONCERNS FOR HYALURONIC ACID THERAPY IN KNEE OSTEOARTHRITIS
7.4.2 SHORT-TERM BENEFITS WITH LIMITED LASTING EFFICACY OF HYALURONIC ACID FOR KNEE OA
8 EUROPE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY PRODUCT TYPE
8.1 OVERVIEW
8.2 SINGLE INJECTION (MONOPHASIC)
8.3 THREE-INJECTION REGIMEN (TRIPHASIC)
8.4 FIVE-INJECTION REGIMEN (PENTAPHASIC)
9 EUROPE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY FORMULATION
9.1 OVERVIEW
9.2 CROSS-LINKED HYALURONIC ACID
9.3 NON-CROSS-LINKED HYALURONIC ACID
10 EUROPE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY MOLECULAR WEIGHT
10.1 OVERVIEW
10.2 HIGH MOLECULAR WEIGHT (HMW) HA
10.3 LOW MOLECULAR WEIGHT (LMW) HA
10.4 INTERMEDIATE MOLECULAR WEIGHT HA
11 EUROPE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY TREATMENT GOALS
11.1 OVERVIEW
11.2 VISCOSUPPLEMENTATION
11.3 ANTI-INFLAMMATORY & PAIN REDUCTION
11.4 CARTILAGE PROTECTION & REGENERATION
12 EUROPE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY END USER
12.1 OVERVIEW
12.2 HOSPITALS
12.3 ORTHOPEDIC CLINICS
12.4 AMBULATORY SURGICAL CENTERS
12.5 SPECIALTY PAIN MANAGEMENT CENTERS
13 EUROPE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY DISTRIBUTION CHANNEL
13.1 OVERVIEW
13.2 DIRECT TENDER
13.3 RETAIL & ONLINE PHARMACIES
13.4 OTHERS
14 EUROPE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY REGION
14.1 EUROPE
14.1.1 GERMANY
14.1.2 FRANCE
14.1.3 U.K.
14.1.4 ITALY
14.1.5 SPAIN
14.1.6 RUSSIA
14.1.7 TURKEY
14.1.8 NETHERLANDS
14.1.9 SWITZERLAND
14.1.10 POLAND
14.1.11 BELGIUM
14.1.12 SWEDEN
14.1.13 AUSTRIA
14.1.14 NORWAY
14.1.15 DENMARK
14.1.16 REST OF EUROPE
15 EUROPE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT: COMPANY LANDSCAPE
15.1 COMPANY SHARE ANALYSIS: GLOBAL
16 SWOT ANALYSIS
17 COMPANY PROFILE
17.1 LG CHEM
17.1.1 COMPANY SNAPSHOT
17.1.2 REVENUE ANALYSIS
17.1.3 COMPANY SHARE ANALYSIS
17.1.4 PRODUCT PORTFOLIO
17.1.5 RECENT DEVELOPMENTS
17.2 BIOVENTUS
17.2.1 COMPANY SNAPSHOT
17.2.2 REVENUE ANALYSIS
17.2.3 COMPANY SHARE ANALYSIS
17.2.4 PRODUCT PORTFOLIO
17.2.5 RECENT DEVELOPMENT
17.3 SEIKAGAKU CORPORATION
17.3.1 COMPANY SNAPSHOT
17.3.2 REVENUE ANALYSIS
17.3.3 COMPANY SHARE ANALYSIS
17.3.4 PRODUCT PORTFOLIO
17.3.5 RECENT DEVELOPMENT
17.4 SANOFI
17.4.1 COMPANY SNAPSHOT
17.4.2 REVENUE ANALYSIS
17.4.3 COMPANY SHARE ANALYSIS
17.4.4 PRODUCT PORTFOLIO
17.4.5 RECENT DEVELOPMENT
17.5 ANIKA THERAPEUTICS, INC.
17.5.1 COMPANY SNAPSHOT
17.5.2 REVENUE ANALYSIS
17.5.3 COMPANY SHARE ANALYSIS
17.5.4 PRODUCT PORTFOLIO
17.5.5 RECENT DEVELOPMENT
17.6 EUPRAXIA PHARMACEUTICALS
17.6.1 COMPANY SNAPSHOT
17.6.2 REVENUE ANALYSIS
17.6.3 PRODUCT PORTFOLIO
17.6.4 RECENT DEVELOPMENT
17.7 FIDIA FARMACEUTICI S.P.A.
17.7.1 COMPANY SNAPSHOT
17.7.2 REVENUE ANALYSIS
17.7.3 PRODUCT PORTFOLIO
17.7.4 RECENT DEVELOPMENT
17.8 FERRING
17.8.1 COMPANY SNAPSHOT
17.8.2 PRODUCT PORTFOLIO
17.8.3 RECENT DEVELOPMENT
17.9 HANGZHOU SINGCLEAN MEDICAL PRODUCTS CO.,LTD.
17.9.1 COMPANY SNAPSHOT
17.9.2 PRODUCT PORTFOLIO
17.9.3 RECENT DEVELOPMENTS
17.1 HANMI PHARM.CO., LTD
17.10.1 COMPANY SNAPSHOT
17.10.2 REVENUE ANALYSIS
17.10.3 PRODUCT PORTFOLIO
17.10.4 RECENT DEVELOPMENT
17.11 IBSA INSTITUT BIOCHIMIQUE SA.
17.11.1 COMPANY SNAPSHOT
17.11.2 PRODUCT PORTFOLIO
17.11.3 RECENT DEVELOPMENT
17.12 TRB CHEMEDICA INTERNATIONAL SA
17.12.1 COMPANY SNAPSHOT
17.12.2 PRODUCT PORTFOLIO
17.12.3 RECENT DEVELOPMENTS
17.13 VIATRIS INC.
17.13.1 COMPANY SNAPSHOT
17.13.2 REVENUE ANALYSIS
17.13.3 PRODUCT PORTFOLIO
17.13.4 RECENT DEVELOPMENT
17.14 VIRCHOW BIOTECH
17.14.1 COMPANY SNAPSHOT
17.14.2 PRODUCT PORTFOLIO
17.14.3 RECENT DEVELOPMENTS
17.15 ZIMMER BIOMET
17.15.1 COMPANY SNAPSHOT
17.15.2 REVENUE ANALYSIS
17.15.3 PRODUCT PORTFOLIO
17.15.4 RECENT DEVELOPMENT
18 QUESTIONNAIRE
19 RELATED REPORTS
表のリスト
TABLE 1 COMPANY PRODUCT AND ADVANCEMENT IN FORMULATION
TABLE 2 COMPANY PRODUCT AND COMBINATION THERAPY
TABLE 3 PRICE OF THE HYALURONIC ACID INJECTION
TABLE 4 COMPANY PRODUCT AND LASTING EFFICACY
TABLE 5 EUROPE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 6 EUROPE SINGLE INJECTION (MONOPHASIC) IN HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 7 EUROPE THREE-INJECTION REGIMEN (TRIPHASIC) IN HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 8 EUROPE FIVE-INJECTION REGIMEN (PENTAPHASIC IN HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY REGION, 2018-2032 (USD THOUSAND)…
TABLE 9 EUROPE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY FORMULATION, 2018-2032 (USD THOUSAND)
TABLE 10 EUROPE CROSS-LINKED HYALURONIC ACID IN HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 11 EUROPE NON-CROSS-LINKED HYALURONIC ACID IN HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 12 EUROPE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY MOLECULAR WEIGHT, 2018-2032 (USD THOUSAND)
TABLE 13 EUROPE HIGH MOLECULAR WEIGHT (HMW) HA IN HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 14 EUROPE LOW MOLECULAR WEIGHT (LMW) HA IN HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 15 EUROPE INTERMEDIATE MOLECULAR WEIGHT HA IN HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 16 EUROPE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY TREATMENT GOALS, 2018-2032 (USD THOUSAND)
TABLE 17 EUROPE VISCOSUPPLEMENTATION IN HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 18 EUROPE ANTI-INFLAMMATORY & PAIN REDUCTION IN HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 19 EUROPE CARTILAGE PROTECTION & REGENERATION IN HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 20 EUROPE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY TREATMENT GOALS, 2018-2032 (USD THOUSAND)
TABLE 21 EUROPE HOSPITALS IN HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 22 EUROPE ORTHOPEDIC CLINICS IN HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 23 EUROPE AMBULATORY SURGICAL CENTERS IN HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 24 EUROPE SPECIALTY PAIN MANAGEMENT CENTERS IN HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 25 EUROPE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 26 EUROPE DIRECT TENDER IN HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 27 EUROPE RETAIL & ONLINE PHARMACIES IN HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 28 EUROPE OTHERS IN HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 29 EUROPE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY COUNTRY, 2018-2032 (USD THOUSAND)
TABLE 30 EUROPE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 31 EUROPE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY FORMULATION, 2018-2032 (USD THOUSAND)
TABLE 32 EUROPE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY MOLECULAR WEIGHT, 2018-2032 (USD THOUSAND)
TABLE 33 EUROPE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY TREATMENT GOALS, 2018-2032 (USD THOUSAND)
TABLE 34 EUROPE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 35 EUROPE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 36 GERMANY HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 37 GERMANY HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY FORMULATION, 2018-2032 (USD THOUSAND)
TABLE 38 GERMANY HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY MOLECULAR WEIGHT, 2018-2032 (USD THOUSAND)
TABLE 39 GERMANY HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY TREATMENT GOALS, 2018-2032 (USD THOUSAND)
TABLE 40 GERMANY HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 41 GERMANY HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 42 FRANCE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 43 FRANCE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY FORMULATION, 2018-2032 (USD THOUSAND)
TABLE 44 FRANCE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY MOLECULAR WEIGHT, 2018-2032 (USD THOUSAND)
TABLE 45 FRANCE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY TREATMENT GOALS, 2018-2032 (USD THOUSAND)
TABLE 46 FRANCE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 47 FRANCE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 48 U.K. HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 49 U.K. HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY FORMULATION, 2018-2032 (USD THOUSAND)
TABLE 50 U.K. HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY MOLECULAR WEIGHT, 2018-2032 (USD THOUSAND)
TABLE 51 U.K. HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY TREATMENT GOALS, 2018-2032 (USD THOUSAND)
TABLE 52 U.K. HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 53 U.K. HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 54 ITALY HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 55 ITALY HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY FORMULATION, 2018-2032 (USD THOUSAND)
TABLE 56 ITALY HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY MOLECULAR WEIGHT, 2018-2032 (USD THOUSAND)
TABLE 57 ITALY HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY TREATMENT GOALS, 2018-2032 (USD THOUSAND)
TABLE 58 ITALY HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 59 ITALY HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 60 SPAIN HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 61 SPAIN HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY FORMULATION, 2018-2032 (USD THOUSAND)
TABLE 62 SPAIN HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY MOLECULAR WEIGHT, 2018-2032 (USD THOUSAND)
TABLE 63 SPAIN HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY TREATMENT GOALS, 2018-2032 (USD THOUSAND)
TABLE 64 SPAIN HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 65 SPAIN HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 66 RUSSIA HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 67 RUSSIA HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY FORMULATION, 2018-2032 (USD THOUSAND)
TABLE 68 RUSSIA HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY MOLECULAR WEIGHT, 2018-2032 (USD THOUSAND)
TABLE 69 RUSSIA HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY TREATMENT GOALS, 2018-2032 (USD THOUSAND)
TABLE 70 RUSSIA HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 71 RUSSIA HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 72 TURKEY HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 73 TURKEY HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY FORMULATION, 2018-2032 (USD THOUSAND)
TABLE 74 TURKEY HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY MOLECULAR WEIGHT, 2018-2032 (USD THOUSAND)
TABLE 75 TURKEY HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY TREATMENT GOALS, 2018-2032 (USD THOUSAND)
TABLE 76 TURKEY HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 77 TURKEY HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 78 NETHERLANDS HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 79 NETHERLANDS HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY FORMULATION, 2018-2032 (USD THOUSAND)
TABLE 80 NETHERLANDS HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY MOLECULAR WEIGHT, 2018-2032 (USD THOUSAND)
TABLE 81 NETHERLANDS HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY TREATMENT GOALS, 2018-2032 (USD THOUSAND)
TABLE 82 NETHERLANDS HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 83 NETHERLANDS HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 84 SWITZERLAND HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 85 SWITZERLAND HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY FORMULATION, 2018-2032 (USD THOUSAND)
TABLE 86 SWITZERLAND HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY MOLECULAR WEIGHT, 2018-2032 (USD THOUSAND)
TABLE 87 SWITZERLAND HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY TREATMENT GOALS, 2018-2032 (USD THOUSAND)
TABLE 88 SWITZERLAND HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 89 SWITZERLAND HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 90 POLAND HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 91 POLAND HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY FORMULATION, 2018-2032 (USD THOUSAND)
TABLE 92 POLAND HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY MOLECULAR WEIGHT, 2018-2032 (USD THOUSAND)
TABLE 93 POLAND HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY TREATMENT GOALS, 2018-2032 (USD THOUSAND)
TABLE 94 POLAND HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 95 POLAND HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 96 BELGIUM HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 97 BELGIUM HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY FORMULATION, 2018-2032 (USD THOUSAND)
TABLE 98 BELGIUM HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY MOLECULAR WEIGHT, 2018-2032 (USD THOUSAND)
TABLE 99 BELGIUM HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY TREATMENT GOALS, 2018-2032 (USD THOUSAND)
TABLE 100 BELGIUM HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 101 BELGIUM HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 102 SWEDEN HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 103 SWEDEN HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY FORMULATION, 2018-2032 (USD THOUSAND)
TABLE 104 SWEDEN HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY MOLECULAR WEIGHT, 2018-2032 (USD THOUSAND)
TABLE 105 SWEDEN HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY TREATMENT GOALS, 2018-2032 (USD THOUSAND)
TABLE 106 SWEDEN HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 107 SWEDEN HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 108 AUSTRIA HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 109 AUSTRIA HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY FORMULATION, 2018-2032 (USD THOUSAND)
TABLE 110 AUSTRIA HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY MOLECULAR WEIGHT, 2018-2032 (USD THOUSAND)
TABLE 111 AUSTRIA HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY TREATMENT GOALS, 2018-2032 (USD THOUSAND)
TABLE 112 AUSTRIA HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 113 AUSTRIA HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 114 NORWAY HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 115 NORWAY HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY FORMULATION, 2018-2032 (USD THOUSAND)
TABLE 116 NORWAY HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY MOLECULAR WEIGHT, 2018-2032 (USD THOUSAND)
TABLE 117 NORWAY HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY TREATMENT GOALS, 2018-2032 (USD THOUSAND)
TABLE 118 NORWAY HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 119 NORWAY HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 120 DENMARK HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
TABLE 121 DENMARK HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY FORMULATION, 2018-2032 (USD THOUSAND)
TABLE 122 DENMARK HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY MOLECULAR WEIGHT, 2018-2032 (USD THOUSAND)
TABLE 123 DENMARK HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY TREATMENT GOALS, 2018-2032 (USD THOUSAND)
TABLE 124 DENMARK HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 125 DENMARK HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 126 REST OF EUROPE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY PRODUCT TYPE, 2018-2032 (USD THOUSAND)
図表一覧
FIGURE 1 EUROPE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT: SEGMENTATION
FIGURE 2 EUROPE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT: DATA TRIANGULATION
FIGURE 3 EUROPE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT: DROC ANALYSIS
FIGURE 4 EUROPE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT: REGIONAL MARKET ANALYSIS
FIGURE 5 EUROPE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT: COMPANY RESEARCH ANALYSIS
FIGURE 6 EUROPE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT: LIFELINE CURVE
FIGURE 7 EUROPE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT: INTERVIEW DEMOGRAPHICS
FIGURE 8 EUROPE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT: DBMR MARKET POSITION GRID
FIGURE 9 EUROPE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT: MARKET END USER COVERAGE GRID
FIGURE 10 EUROPE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT: VENDOR SHARE ANALYSIS
FIGURE 11 EUROPE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT: SEGMENTATION
FIGURE 12 THREE SEGMENTS COMPRISE THE EUROPE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT, BY PRODUCT TYPE
FIGURE 13 EUROPE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT EXECUTIVE SUMMARY
FIGURE 14 STRATEGIC DECISIONS
FIGURE 15 RISING PREVALENCE OF KNEE OSTEOARTHRITIS IS EXPECTED TO DRIVE THE GROWTH OF EUROPE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT FROM 2025 TO 2032
FIGURE 16 THE SINGLE INJECTION (MONOPHASIC) SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE EUROPE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT IN 2025 - 2032
FIGURE 17 NORTH AMERICA IS EXPECTED TO DOMINATE THE EUROPE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT AND ASIA-PACIFIC IS EXPECTED TO GROW WITH THE HIGHEST CAGR IN THE FORECAST PERIOD OF 2025 TO 2032
FIGURE 18 ASIA-PACIFIC IS THE FASTEST-GROWING MARKET FOR EUROPE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT IN THE FORECAST PERIOD OF 2025 TO 2032
FIGURE 19 DROC ANALYSIS
FIGURE 20 EUROPE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT: BY PRODUCT TYPE, 2024
FIGURE 21 EUROPE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT: BY PRODUCT TYPE, 2025-2032 (USD THOUSAND)
FIGURE 22 EUROPE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT: BY PRODUCT TYPE, CAGR (2025-2032)
FIGURE 23 EUROPE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT: BY PRODUCT TYPE, LIFELINE CURVE
FIGURE 24 EUROPE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT: BY FORMULATION, 2024
FIGURE 25 EUROPE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT: BY FORMULATION, 2025-2032 (USD THOUSAND)
FIGURE 26 EUROPE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT: BY FORMULATION, CAGR (2025-2032)
FIGURE 27 EUROPE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT: BY FORMULATION, LIFELINE CURVE
FIGURE 28 EUROPE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT: BY MOLECULAR WEIGHT, 2024
FIGURE 29 EUROPE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT: BY MOLECULAR WEIGHT, 2025-2032 (USD THOUSAND)
FIGURE 30 EUROPE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT: BY MOLECULAR WEIGHT, CAGR (2025-2032)
FIGURE 31 EUROPE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT: BY MOLECULAR WEIGHT, LIFELINE CURVE
FIGURE 32 EUROPE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT: BY TREATMENT GOALS, 2024
FIGURE 33 EUROPE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT: BY TREATMENT GOALS, 2025-2032 (USD THOUSAND)
FIGURE 34 EUROPE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT: BY TREATMENT GOALS, CAGR (2025-2032)
FIGURE 35 EUROPE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT: BY TREATMENT GOALS, LIFELINE CURVE
FIGURE 36 EUROPE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT: BY END USER, 2024
FIGURE 37 EUROPE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT: BY END USER, 2025-2032 (USD THOUSAND)
FIGURE 38 EUROPE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT: BY END USER, CAGR (2025-2032)
FIGURE 39 EUROPE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT: BY END USER, LIFELINE CURVE
FIGURE 40 EUROPE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT: BY DISTRIBUTION CHANNEL, 2024
FIGURE 41 EUROPE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT: BY DISTRIBUTION CHANNEL, 2025-2032 (USD THOUSAND)
FIGURE 42 EUROPE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT: BY DISTRIBUTION CHANNEL, CAGR (2025-2032)
FIGURE 43 EUROPE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 44 EUROPE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT: SNAPSHOT (2024)
FIGURE 45 EUROPE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT: COMPANY SHARE 2024 (%)
FIGURE 46 NORTH AMERICA HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT: COMPANY SHARE 2024 (%)
FIGURE 47 EUROPE HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT: COMPANY SHARE 2024 (%)
FIGURE 48 ASIA-PACIFIC HYALURONIC ACID MARKET FOR KNEE OSTEOARTHRITIS TREATMENT: COMPANY SHARE 2024 (%)

調査方法
データ収集と基準年分析は、大規模なサンプル サイズのデータ収集モジュールを使用して行われます。この段階では、さまざまなソースと戦略を通じて市場情報または関連データを取得します。過去に取得したすべてのデータを事前に調査および計画することも含まれます。また、さまざまな情報ソース間で見られる情報の不一致の調査も含まれます。市場データは、市場統計モデルと一貫性モデルを使用して分析および推定されます。また、市場シェア分析と主要トレンド分析は、市場レポートの主要な成功要因です。詳細については、アナリストへの電話をリクエストするか、お問い合わせをドロップダウンしてください。
DBMR 調査チームが使用する主要な調査方法は、データ マイニング、データ変数が市場に与える影響の分析、および一次 (業界の専門家) 検証を含むデータ三角測量です。データ モデルには、ベンダー ポジショニング グリッド、市場タイムライン分析、市場概要とガイド、企業ポジショニング グリッド、特許分析、価格分析、企業市場シェア分析、測定基準、グローバルと地域、ベンダー シェア分析が含まれます。調査方法について詳しくは、お問い合わせフォームから当社の業界専門家にご相談ください。
カスタマイズ可能
Data Bridge Market Research は、高度な形成的調査のリーダーです。当社は、既存および新規のお客様に、お客様の目標に合致し、それに適したデータと分析を提供することに誇りを持っています。レポートは、対象ブランドの価格動向分析、追加国の市場理解 (国のリストをお問い合わせください)、臨床試験結果データ、文献レビュー、リファービッシュ市場および製品ベース分析を含めるようにカスタマイズできます。対象競合他社の市場分析は、技術ベースの分析から市場ポートフォリオ戦略まで分析できます。必要な競合他社のデータを、必要な形式とデータ スタイルでいくつでも追加できます。当社のアナリスト チームは、粗い生の Excel ファイル ピボット テーブル (ファクト ブック) でデータを提供したり、レポートで利用可能なデータ セットからプレゼンテーションを作成するお手伝いをしたりすることもできます。