Europe Intrauterine Contraceptive Devices Market Size, Share, and Trends Analysis Report
Market Size in USD Billion
CAGR :
% 
 USD
225.00 Million
USD
326.56 Million
2024
2032
USD
225.00 Million
USD
326.56 Million
2024
2032
| 2025 –2032 | |
| USD 225.00 Million | |
| USD 326.56 Million | |
|
|
|
|
Europe Intrauterine Contraceptive Devices Market Segmentation By Type of Product (Hormonal, IUCD and Copper IUCD), End Use (Hospitals, Gynecology Clinics, Community Healthcare and Others) - Industry Trends and Forecast to 2032
Intrauterine Contraceptive Devices Market Size
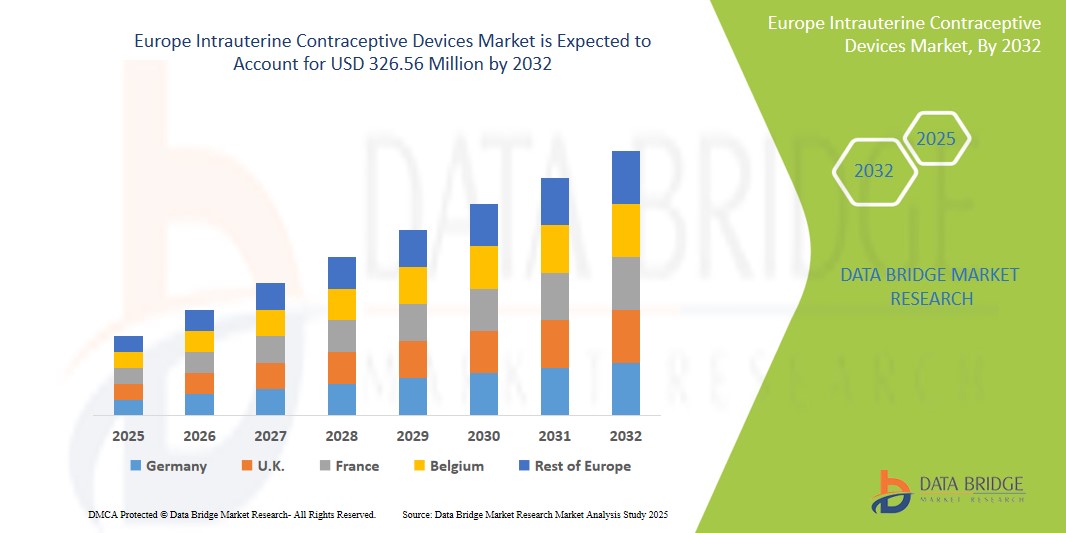
- The Europe Intrauterine Contraceptive Devices Market was valued atUSD225 million in 2024 and is expected to reachUSD326.56 million by 2032, at aCAGR of 3.66%During the forecast period.
- The Europe Intrauterine Contraceptive Devices Market is primarily driven by several key factors. These include the increasing awareness and adoption of long-acting reversible contraceptives (LARCs), favorable government initiatives promoting family planning, and rising concerns regarding unintended pregnancies. Furthermore, the advancement in device design, enhanced safety and efficacy profiles, and greater accessibility through healthcare infrastructure improvements are contributing significantly to market growth across the region.
Europe Intrauterine Contraceptive Devices Market Analysis
- Intrauterine Contraceptive Devices play a crucial role in modern reproductive health management across Europe. These devices offer a long-term, reversible, and highly effective method of contraception, which reduces the reliance on daily or short-term contraceptive methods. Their use significantly contributes to family planning initiatives and helps in reducing unintended pregnancies, thereby improving overall women's health outcomes across the region.
- The demand for Intrauterine Contraceptive Devices in Europe is primarily driven by factors such as increasing awareness about long-acting contraceptive options, the rising need for effective birth control methods, and supportive government policies promoting reproductive health. Additionally, a growing emphasis on women’s empowerment, delayed childbearing, and increased healthcare access has led to higher adoption rates. Technological innovations—such as hormone-releasing IUDs and improved biocompatible materials—are further enhancing patient comfort and device efficacy, accelerating market growth.
- Europe holds a significant position in the global Intrauterine Contraceptive Devices market, supported by a well-established healthcare infrastructure, early adoption of advanced medical technologies, and comprehensive public health programs. Countries such as Germany, France, and the UK are leading the regional market due to proactive healthcare initiatives, widespread educational campaigns on reproductive health, and favorable reimbursement frameworks.
- Regulatory support from European medical agencies, along with increasing investments in women-centric healthcare solutions, are further propelling the market. Moreover, a shift toward personalized reproductive health strategies, including tailored contraceptive counseling and follow-up care, is helping to ensure better patient satisfaction and long-term use, thereby driving sustained market expansion across the region.
Report ScopeIntrauterine Contraceptive DevicesMarket Segmentation
|
Attributes |
Intrauterine Contraceptive DevicesKey Market Insights |
|
Segments Covered |
|
|
Countries Covered |
Europe
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include import export analysis, production capacity overview, production consumption analysis, price trend analysis, climate change scenario, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Intrauterine Contraceptive Devices Market Trends
“Integration of Smart Technology and AI-Driven Health Insights”
- A prominent trend in the Europe Intrauterine Contraceptive Devices Market is the integration of smart technology—including embedded sensors and AI-based analytics—to deliver real-time reproductive health data and predictive insights. These innovations aim to enhance contraceptive monitoring, improve device safety, and enable personalized healthcare. Advanced IUDs are now being designed to collect user data, which is analyzed through machine learning algorithms to support individualized contraceptive guidance and optimize long-term outcomes.
- For instance, European health tech companies are actively exploring digital contraceptive tools and AI-enabled platforms that track menstrual health, ovulation patterns, and user feedback, contributing to better contraceptive management and adherence.
- Another growing trend is the integration of intrauterine contraceptive devices with broader digital health ecosystems, such as mobile health applications and electronic health records (EHRs). This interconnected approach ensures that both patients and healthcare professionals have continuous, centralized access to reproductive health data, improving clinical decision-making and patient engagement. Additionally, the adoption of cloud-based platforms for secure data storage and remote monitoring capabilities is supporting efficient, long-term follow-up—especially critical for users in rural or resource-limited regions.
Intrauterine Contraceptive Devices Market Dynamics
Driver
“Rising Awareness of Reproductive Health and Government-Backed Family Planning Initiatives”
- One of the key drivers fueling the growth of the Europe Intrauterine Contraceptive Devices Market is the increasing awareness of reproductive health and the importance of planned parenthood. Educational campaigns, public health programs, and growing acceptance of long-acting reversible contraceptives (LARCs) have contributed to greater IUD adoption across various European demographics.
- National governments and health agencies across Europe are implementing family planning initiatives, offering subsidized or free contraceptive services through public healthcare systems. These efforts aim to reduce unplanned pregnancies and promote reproductive autonomy, particularly among adolescents and underserved populations.
- The shift in social attitudes toward contraception—supported by inclusive sexual education programs and NGO-led advocacy—has also played a vital role in destigmatizing intrauterine devices. This has led to greater patient confidence, especially among younger women and first-time users.
- Technological advancements in IUD design, including non-hormonal options, smaller sizes, and lower complication rates, further reinforce the adoption trend. Healthcare professionals now more frequently recommend IUDs due to their efficacy, reversibility, and low maintenance compared to short-term alternatives.
- For instance, The UK’s National Health Service (NHS) includes intrauterine devices in its publicly funded contraception options, making them widely accessible at no direct cost to patients.
- In France, government-sponsored campaigns have emphasized the safety and effectiveness of IUDs, contributing to a steady rise in adoption among women aged 20–35.
- Germany has integrated reproductive health awareness into its national curriculum, encouraging informed contraceptive choices among young adults.
Opportunity
“Expanding Access to Contraceptive Solutions in Emerging European Markets”
- A significant opportunity in the Europe Intrauterine Contraceptive Devices Market lies in expanding access to contraceptive solutions in emerging European markets. As countries in Eastern and Southern Europe continue to improve their healthcare infrastructure and increase access to family planning services, there is a growing demand for effective, long-term contraceptive options like intrauterine devices (IUDs).
- The rising economic stability and improvement in healthcare policies in countries such as Poland, Hungary, and Romania have created favorable environments for the adoption of modern contraceptive solutions. As these countries align with European Union health standards, there is greater emphasis on improving reproductive health services and supporting affordable family planning methods.
- Government-backed health programs and increased focus on women's health in emerging markets are expected to drive IUD adoption. In addition, collaborations with local governments and healthcare organizations will facilitate the introduction of subsidized contraceptive products, making them accessible to a broader population, particularly in rural and underserved areas.
- The growing recognition of IUDs as a cost-effective contraceptive method, coupled with international support for reproductive health rights, is further spurring demand for these devices in regions that historically had limited access to such healthcare options.
- For instance, In Poland, the government has introduced subsidies for long-term contraceptive methods, leading to an increase in IUD usage.
- Romania has launched initiatives to integrate family planning into public health services, contributing to growing demand for IUDs as part of broader reproductive health programs.
- Bulgaria is seeing rising demand for contraceptives due to a combination of EU funding for healthcare and an increased focus on gender equality and women’s health.
Restraint/Challenge
“Cultural and Socioeconomic Barriers to IUD Adoption”
- A significant restraint in the Europe Intrauterine Contraceptive Devices Market is the cultural and socioeconomic barriers that limit widespread adoption of IUDs, especially in certain regions. In some European countries, cultural attitudes towards contraception, especially long-term methods like IUDs, can deter women from choosing these options due to perceptions of discomfort, fear of side effects, or lack of awareness about the benefits.
- In more conservative communities, there is sometimes hesitation to adopt intrauterine devices, as they are often seen as a method that may be unsuitable for younger women or those who have not had children. This perception persists even though IUDs are increasingly being recommended for women of all reproductive histories.
- Additionally, socioeconomic factors such as lack of access to quality healthcare, financial constraints, or lack of comprehensive insurance coverage in certain regions limit the ability of women to afford or obtain IUDs. Although IUDs are cost-effective in the long term, the initial cost may be prohibitive for some individuals, particularly in countries where healthcare subsidies or insurance coverage for contraception are limited.
- Some regions with limited access to reproductive health education and family planning services also face higher rates of misinformation or misunderstanding about IUDs. This can create significant barriers to adoption, despite the availability of effective and safe contraceptive options.
- For instance, In Italy and some Eastern European countries, there is often a preference for short-term contraceptive methods like birth control pills due to cultural perceptions about the invasiveness of IUDs.
- In Greece, certain rural areas face limited access to affordable family planning services, which results in lower adoption of long-acting contraceptive methods, including IUDs.
- In Spain, women in lower socioeconomic brackets sometimes face difficulty affording the upfront cost of IUDs, despite their long-term cost-efficiency.
Intrauterine Contraceptive Devices Market Scope
The market is segmented into three notable segments based on type of product and end User .
|
Segmentation |
Sub-Segmentation |
|
By Type of Product |
|
|
By end user |
|
In 2025, the Hormonal is projected to dominate the market with a largest share in the type of product segment
The Hormonal is expected to lead the Europe Intrauterine Contraceptive Devices Market with the largest share of 41.42% in 2025.This dominance is driven by the growing preference for long-term, effective, and reversible contraception options among European women.
The Hospitals is expected to account for the largest share during the forecast period in end use market
In 2025, the Hospitals segment is expected to dominate the market with the largest market share of 51.11% due to its high prevalence and demand for precision. This is attributed to the increasing number of hospital-based procedures requiring advanced medical devices and the demand for high-quality, accurate care.
Intrauterine Contraceptive Devices Market Regional Analysis
“Germany is the Dominant Country in the Intrauterine Contraceptive Devices Market”
- Germany dominates the European Intrauterine Contraceptive Devices Market, accounting for the largest share due to its advanced healthcare system, high levels of healthcare expenditure, and early adoption of medical innovations.
- The growing awareness of long-term contraceptive methods and the increasing demand for effective family planning solutions contribute significantly to market growth.
- Major players like Bayer and Merck, headquartered in Germany, provide a wide range of intrauterine contraceptive devices that cater to varying consumer preferences and regulatory standards across Europe.
- Government initiatives to improve access to reproductive healthcare and provide support for family planning further reinforce Germany’s leadership in the European Intrauterine Contraceptive Devices Market.
“France is Projected to Register the Highest Growth Rate”
- France is expected to register the fastest growth in the European Intrauterine Contraceptive Devices Market, driven by its robust public healthcare system and rising awareness regarding the benefits of intrauterine contraceptives.
- The increase in government support for preventive reproductive health services, coupled with the growing demand for reversible contraception, is fueling market expansion.
- The rising focus on women’s health and fertility preservation, along with the growing number of healthcare campaigns, is accelerating the adoption of intrauterine contraceptive devices in France.
- Collaboration between healthcare providers and insurance companies to make contraceptive options more accessible is further boosting the demand for these devices across the country.
Intrauterine Contraceptive Devices Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, Europe presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- Bayer AG (Germany)
- Merck & Co., Inc. (Germany/USA)
- CooperSurgical, Inc. (USA)
- Pregna International Limited (India)
- HLL Lifecare Limited (India)
- Medisafe Distribution, Inc. (USA)
- Smith & Nephew (UK)
- Teva Pharmaceutical Industries Ltd. (Israel)
- Prosan (Switzerland)
- Apoteket AB (Sweden)
Latest Developments in Europe Intrauterine Contraceptive Devices Market
- In May 2023, Sebela Pharmaceuticals announced positive Phase 3 study results for its investigational Copper 175 mm² IUD. This next-generation, low-dose, hormone-free copper IUD has achieved a 99% efficacy rate over three years, with a Pearl Index of 0.96. The study, conducted at 42 centers across the U.S., found that the IUD was well-tolerated, with a reported 98.8% placement success rate. Both clinicians and participants provided positive feedback regarding the IUD.
- In August 2022, Bayer AG received FDA approval for a supplemental new drug application (sNDA) for Mirena IUD, extending its period of use to up to eight years. This approval is intended to ensure that women have access to the contraceptive options needed at various stages of their reproductive life
- In January 2024, Medicines360, a global nonprofit in women's health, and DKT WomanCare, a global distributor of contraceptive products, partnered to expand access to AVIBELA. AVIBELA is a cost-effective, high-quality hormonal IUD with over 99% effectiveness. This collaboration aims to increase awareness, accessibility, and usage of this highly effective contraceptive option
- In June 2023, Pregna International Limited, a company specializing in women's reproductive health, received an initial private equity investment of USD 16 million (₹130 crore) from India Life Sciences Fund III (ILSF III), managed by the private equity firm InvAscent. This investment aims to fuel Pregna's growth and empower them to address the evolving needs of women globally
SKU-
世界初のマーケットインテリジェンスクラウドに関するレポートにオンラインでアクセスする
- インタラクティブなデータ分析ダッシュボード
- 成長の可能性が高い機会のための企業分析ダッシュボード
- カスタマイズとクエリのためのリサーチアナリストアクセス
- インタラクティブなダッシュボードによる競合分析
- 最新ニュース、更新情報、トレンド分析
- 包括的な競合追跡のためのベンチマーク分析のパワーを活用
調査方法
データ収集と基準年分析は、大規模なサンプル サイズのデータ収集モジュールを使用して行われます。この段階では、さまざまなソースと戦略を通じて市場情報または関連データを取得します。過去に取得したすべてのデータを事前に調査および計画することも含まれます。また、さまざまな情報ソース間で見られる情報の不一致の調査も含まれます。市場データは、市場統計モデルと一貫性モデルを使用して分析および推定されます。また、市場シェア分析と主要トレンド分析は、市場レポートの主要な成功要因です。詳細については、アナリストへの電話をリクエストするか、お問い合わせをドロップダウンしてください。
DBMR 調査チームが使用する主要な調査方法は、データ マイニング、データ変数が市場に与える影響の分析、および一次 (業界の専門家) 検証を含むデータ三角測量です。データ モデルには、ベンダー ポジショニング グリッド、市場タイムライン分析、市場概要とガイド、企業ポジショニング グリッド、特許分析、価格分析、企業市場シェア分析、測定基準、グローバルと地域、ベンダー シェア分析が含まれます。調査方法について詳しくは、お問い合わせフォームから当社の業界専門家にご相談ください。
カスタマイズ可能
Data Bridge Market Research は、高度な形成的調査のリーダーです。当社は、既存および新規のお客様に、お客様の目標に合致し、それに適したデータと分析を提供することに誇りを持っています。レポートは、対象ブランドの価格動向分析、追加国の市場理解 (国のリストをお問い合わせください)、臨床試験結果データ、文献レビュー、リファービッシュ市場および製品ベース分析を含めるようにカスタマイズできます。対象競合他社の市場分析は、技術ベースの分析から市場ポートフォリオ戦略まで分析できます。必要な競合他社のデータを、必要な形式とデータ スタイルでいくつでも追加できます。当社のアナリスト チームは、粗い生の Excel ファイル ピボット テーブル (ファクト ブック) でデータを提供したり、レポートで利用可能なデータ セットからプレゼンテーションを作成するお手伝いをしたりすることもできます。