ヨーロッパ屈折矯正手術機器市場
Market Size in USD Billion
CAGR :
% 
 USD
422.98 Million
USD
746.40 Million
2021
2029
USD
422.98 Million
USD
746.40 Million
2021
2029
| 2022 –2029 | |
| USD 422.98 Million | |
| USD 746.40 Million | |
|
|
|
ヨーロッパ屈折矯正手術機器市場、製品タイプ別(レーザー、有水晶体眼内レンズ(IOL)、アベロメーター/波面アベロメトリー、手術器具および付属品、屈折矯正手術キット、瞳孔径計、エピケラトーム、マイクロケラトーム、サーモケラトプラスティ、輪部弛緩切開キットなど)、手術タイプ別(LASIK(レーザー角膜切開術、フォトレフラクティブ角膜切除術(PRK)、有水晶体眼内レンズ(IOL)、乱視角膜切開術(AK)、自動ラメラ角膜形成術(ALK)、角膜内リング(INTACS)、レーザーサーマル角膜形成術(LTK)、導電性角膜形成術(CK)、放射状角膜切開術(RK)など)、用途別(近視(ミオピア)、遠視(ハイオピア)、乱視と老眼)、エンドユーザー(病院、専門クリニック、外来手術センターなど)、流通チャネル(直接入札、サードパーティの販売代理店など)の業界動向と2029年までの予測。
市場の定義と洞察
屈折矯正手術装置は、近視、遠視、老眼、乱視などの屈折異常を改善または矯正するために使用されます。これらの装置には、エキシマ レーザー、YAG レーザー、マイクロケラトーム、フェムト秒レーザーなどがあります。屈折矯正手術により、眼鏡やコンタクト レンズへの依存度が大幅に軽減されます。市場では、視力障害の治療にさまざまな屈折矯正装置が使用されています。

屈折異常は、角膜または眼球の形状が不適切であるために発生します。屈折矯正手術には、高度なレーザー、レーシック治療、光屈折角膜切除術、有水晶体眼内レンズやトーリック眼内レンズなどのさまざまなレンズなど、さまざまな屈折矯正手術装置を使用して眼球または角膜の形状を変更することが含まれます。
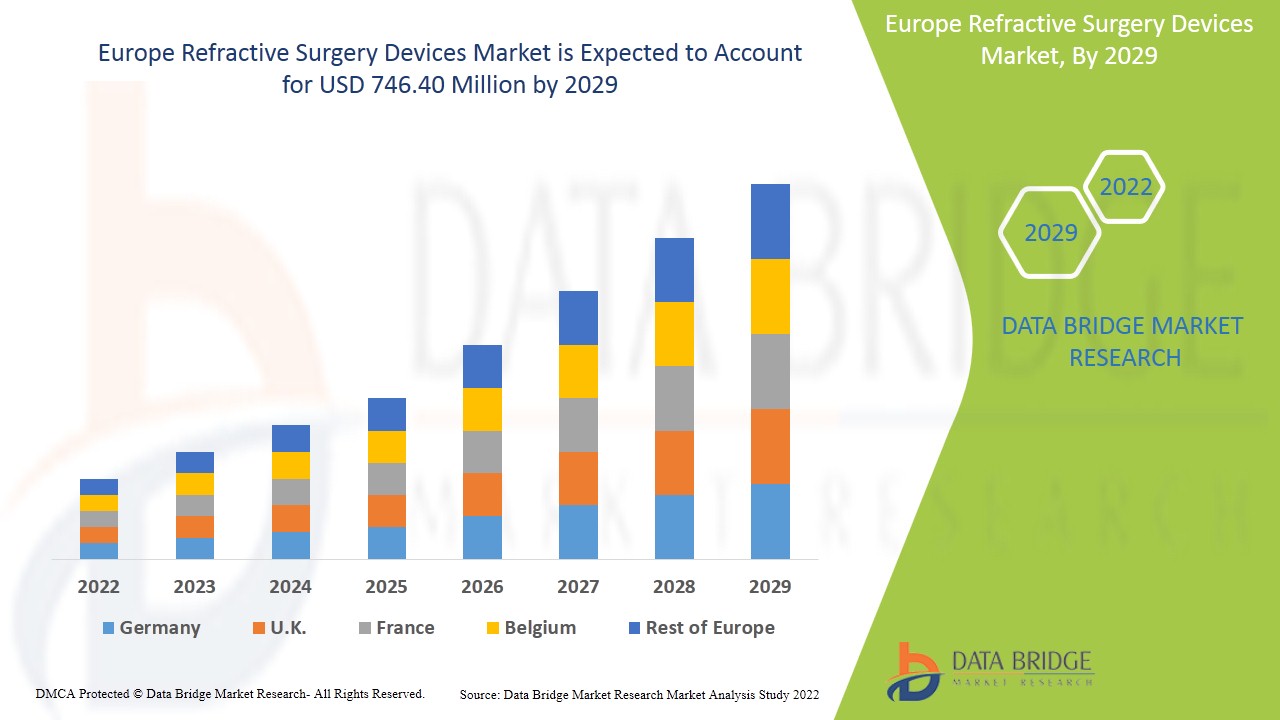
屈折矯正手術装置市場は、2022年から2029年の予測期間に市場成長が見込まれています。データブリッジマーケットリサーチは、市場は2022年から2029年の予測期間に7.6%のCAGRで成長し、2021年の4億2,298万米ドルから2029年には7億4,640万米ドルに達すると分析しています。
|
レポートメトリック |
詳細 |
|
予測期間 |
2022年から2029年 |
|
基準年 |
2021 |
|
歴史的な年 |
2020 (2019 - 2014 にカスタマイズ可能) |
|
定量単位 |
収益(百万米ドル) |
|
対象セグメント |
製品タイプ別 (レーザー、有水晶体眼内レンズ (IOL)、収差計/波面収差測定器、手術器具および付属品、屈折矯正手術キット、瞳孔径計、エピケラトーム、マイクロケラトーム、サーモケラトプラスティ、輪部弛緩切開キットなど)、手術タイプ別 (LASIK (レーザー角膜切削術、フォトレフラクティブ角膜切除術 (PRK)、有水晶体眼内レンズ (IOL)、乱視角膜切開術 (AK)、自動ラメラ角膜形成術 (ALK)、角膜内リング (INTACS)、レーザーサーマル角膜形成術 (LTK)、導電性角膜形成術 (CK)、放射状角膜切開術 (RK) など)、用途別 (近視 (近視)、遠視 (遠視)、乱視および老眼)、エンドユーザー(病院、専門クリニック、外来手術センターなど)、流通チャネル (直接入札、サードパーティの販売業者など) |
|
対象国 |
ドイツ、フランス、イギリス、イタリア、スペイン、オランダ、ロシア、スイス、ベルギー、トルコ、オーストリア、ノルウェー、ハンガリー、リトアニア、アイルランド、ポーランドその他のヨーロッパ諸国 |
|
対象となる市場プレーヤー |
Tracey Technologies、Bausch + Lomb Incorporated、BD、STAAR SURGICAL、SCHWIND eye-tech-solutions、Hoya Surgical Optics、Johnson & Johnson Services、Inc.、Ophtec BV、Glaukos Corporation、Amplitude Laser、Reichert、Inc.、NIDEK CO.、LTD.、Ziemer Ophthalmic Systems、ROWIAK GmbH、Moria、LENSAR、Inc.、Topcon Canada Inc.(Topcon Corporation の子会社)、Aaren Scientific Inc.、Rayner Intraocular Lenses Limited.、iVIS Technologies、Alcon など |
屈折矯正手術機器市場の市場動向には以下が含まれます。
ドライバー
- 技術進歩の増加
ヘルスケア分野における技術開発の加速は、ここ数年で飛躍的に増加しています。屈折矯正手術装置の技術の進歩は、病気の治療中に痛みがなく、合併症のない治療をサポートします。さらに、さまざまな屈折矯正手術装置の革新とアップグレードは、病気の診断を正確かつ迅速に行うのに役立ちます。屈折矯正手術装置の革新は、病気の治療中に技術ベースの治療ツールの費用対効果も高めます。
例えば、
- Contoura Vision Indiaによると、Contoura Vision手術は眼鏡除去における最新の高度な眼科手術であることがわかりました。これは眼科手術における最も安全な技術的進歩の1つであり、眼鏡の度数を矯正するだけでなく、角膜の不規則性にも効果があります。
- 眼科レーザーセンター組織によると、2017年5月、Visumaxフェムト秒レーザー技術は最も先進的な屈折矯正手術の1つであることが判明しました。この技術は、目の視覚障害を治療することができます。
The increased technological advancements in various refractive surgery devices, such as advancements in lasers variable spot scanning, are expected to drive the refractive surgery devices market. Hence, growing innovation and technological advancement in refractive surgery devices are expected to bolster the market growth during the forecast period.
- Rise in healthcare expenditure
Over the last decade, healthcare expenditure has risen drastically for better patient healthcare service. The U.S. is the largest healthcare market, where the total health expenditure has increased drastically in the last few years. The fundamental purpose behind growing expenditure is to provide appropriate, affordable, and high-quality refractive surgery devices. To promote a healthier population and address the healthcare emergencies in developed and developing countries, respective government bodies and healthcare organizations are taking the initiative under accelerating healthcare expenditure.
For instance,
- According to the Health Affairs Organisation, U.S. health care spending has increased 9.7% to reach USD 4.1 trillion in 2020, which is a much faster rate than that seen in 2019
- According to the U.K. government, in 2020, the government have provided nearly GBP 250 million, which is about USD 300 million, to digitalize and advance diagnostics care across the NHS (National Health Service) using the latest technology. This funding has been allocated specifically for technological improvements to the NHS diagnostic services to detect and begin treating health conditions as early as possible
- The National Free Diagnostic Service Initiative has been rolled out as part of the National Health Mission by the Government of India. This was important to provide comprehensive and quality healthcare free of cost under one roof. With this initiative by the Indian government, several states have attempted several models to ensure the availability of diagnostics in public health facilities
Growing healthcare expenditure is also beneficial for further economic growth as well as healthcare sector growth. It significantly affects the development of new diagnostic tests and new surgical tools. Hence, huge health care expenditure is a favorable factor for the market's growth.
Opportunity
- Achievements in LASIK surgeries
The LASIK success rate or LASIK outcomes are well understood, with thousands of clinical studies looking at visual acuity and patient satisfaction. Recent research reported 99 percent of patients achieve better than 20/40 vision, and more than 90 percent achieve 20/20 or better. Furthermore, LASIK has an unprecedented 96 percent patient satisfaction rate, the highest of any elective procedure.
For instance,
- A 2016 study in the Journal of Cataract & Refractive Surgery found that LASIK has a 96% patient satisfaction rate
As per the article, "LASIK: Know the Rewards and the Risks," 2018
- Eric Donnenfeld, MD, a former president of the American Society of Cataract and Refractive Surgery, completed around 85,000 procedures over his 28-year career
- According to Market Scope, around 10 million Americans have had LASIK surgery since the FDA first approved it in 1999. Around 700,000 LASIK surgeries are done each year, but that's down from a peak of 1.4 million in 2000
Henceforth, the rising number of successful LASIK surgeries worldwide is positively associated with product development, product registration, and product launch. Thus, this is expected to drive the refractive surgery devices market in the coming years.
- Strategic initiatives by market players
An increase in the burden of refractive errors across the world have created more demand for the refractive surgery devices market. The main aim is to improve health management with the development of innovative products and surgery types for quality care with the convenient application. The key players in the refractive surgery devices market have taken strategic initiatives, which include product launches, acquisitions, and many more, and are expected to lead and create more opportunities in the refractive surgery devices market.
For instances,
- In June 2021, Glaukos Corporation received regulatory approval from the Therapeutic Goods Administration (TGA) of Australia for PRESERFLO MicroShunt. PRESERFLO MicroShunt aimed to lessen intraocular pressure (IOP) in the eyes of patients with primary open-angle glaucoma where IOP would remain uncontrollable along with being the maximum tolerated medical therapy and/or where glaucoma progression requires surgery
- In June 2021: Bausch & Lomb Incorporated signed an agreement with Lochan, a company in the Information Technology Services Industry. These companies aimed to develop the next generation of Bausch & Lomb Incorporated's eyeTELLIGENCE clinical decision support software. By utilizing the prevailing cloud-based infrastructure of eyeTELLIGENCE, this software would be developed to allow surgeons to effortlessly combine all factors of the cataract, retinal, and refractive surgery procedures to boost their total practice efficiency
- In March 2021, NIDEK unveiled RT-6100 CB for Windows, optional control software for the RT-6100 Intelligent Refractor, and the TS-610 Tabletop Refraction System. This software attunes to the distinct requirements of patients and operators. Moreover, the software enables refractions, which fulfill social distancing requirements
These many strategic products launched and acquisitions by major companies in the refractive surgery devices market have opened up an opportunity for companies worldwide. These strategies are allowing the companies to strengthen their footprints in the market. Therefore, it is predicted that strategic initiative is the golden opportunity for the market players to accelerate their revenue growth in the market.
Challenges/Restraints
- 処置の利点に関する認識と人々の信頼の欠如
多くの国では、一般の人々は屈折矯正手術や、近視、乱視、老眼などの屈折異常に対するそのさまざまな利点を認識していません。人々は手術が深刻な副作用につながるのではないかと恐れており、市場に大きな課題をもたらすことが予想されます。
例えば、
- 国立衛生研究所(NIH)の2021年の調査によると、人々は手術の合併症を心配し、手術に関する情報が不足しているため、手術を拒否しているという。さらに、調査では、参加者の82.5%が、認識不足のために屈折矯正手術が視力を向上させる可能性があることを知らなかったことが示された。
- 2019年に国際開発途上国医学ジャーナルが発表した研究によると、
- 参加者全体の32.2%が屈折矯正手術は危険だと考えており、9.5%が重篤な合併症を引き起こすと考えていた。
- また、インドの調査では、参加者の64%が屈折矯正手術で視力を改善できることを知らなかったことがわかった。
屈折矯正手術の利点に関する認識の欠如と手術合併症に対する人々の恐怖は、市場の成長にとって大きな課題を生み出すと予想されます。
- 眼科治療のための医療施設の不足
低所得国および中所得国の貧困層は、裕福な層よりも失明や眼科疾患に苦しんでいます。先進国で実施されている進歩および戦略計画は、低所得国では同じようには開始されていません。多くの低所得国では、初期の一次眼科治療として、通常、地域の医療従事者、医師助手、白内障外科医に頼っています。低所得国 (LIC) の眼科は、熱帯気候、脆弱な電力網、劣悪な道路および水道インフラ、限られた診断能力、限られた治療オプションなどの複雑さのため、非常に困難です。
例えば、
- 「低所得国における眼科のための革新的な診断ツール」という記事によると、2020年の報告書では、高所得国における失明や眼疾患の有病率は1,000人あたり0.3人であるのに対し、低所得国では1,000人あたり1.5人と推定されている。これは、低所得国における眼科医療のニーズが満たされていないことを示している。
低所得国におけるもう一つの大きな問題は、眼の痛みやその他の障害に関する人々の認識不足です。多くの調査研究で、低所得国における眼科医療のニーズが高いことが報告されており、満たされていないニーズは多くの医療組織の間で依然として注目を集めています。
例えば、
- 2014年、英国眼科学誌は、政府が開始したビジョン2020計画は、中低所得国を対象とした取り組みが不足しているため、達成には程遠いと報告した。
したがって、低所得国および中所得国における眼科治療のための医療施設の貧弱さは、屈折矯正手術装置市場の成長に対する最大の課題であると考えられています。
COVID-19後の屈折矯正手術機器市場への影響
COVID-19は市場に影響を与えています。パンデミック中のロックダウンと隔離により、大衆の移動が制限されました。その結果、手術の日時が遅れました。したがって、パンデミックはこの市場に悪影響を及ぼしました。
最近の開発
- 2021年7月、ジョンソン・エンド・ジョンソン・ビジョンは、次世代の超音波乳化吸引術(フェイコ)システムであるVERITAS Vision Systemを発売しました。このシステムは、外科医の効率、患者の安全性、快適性の3つの重要な領域を考慮して開発されました。これにより、同社の製品ポートフォリオが拡大しました。
屈折矯正手術機器市場の範囲
屈折矯正手術機器市場は、製品タイプ、手術タイプ、アプリケーション、エンドユーザー、流通チャネルに区分されています。これらのセグメントの成長は、業界のわずかな成長セグメントの分析に役立ち、ユーザーに貴重な市場概要と市場洞察を提供し、コア市場アプリケーションを特定するための戦略的決定を下すのに役立ちます。
製品タイプ
- レーザ
- 有水晶体眼内レンズ(IOL)
- 波面収差測定装置
- 手術器具および付属品
- 屈折矯正手術キット
- 瞳孔径メートル
- エピケラトーム
- マイクロケラトーム
- 熱角膜形成術
- 輪部弛緩切開キット
- その他
製品タイプに基づいて、屈折矯正手術装置市場は、レーザー、有水晶体眼内レンズ(IOL)、収差計/波面収差測定装置、手術器具および付属品、屈折矯正手術キット、瞳孔径計、エピケラトーム、マイクロケラトーム、サーモケラトプラスティ、輪部弛緩切開キットなどに分類されます。
手術の種類
- レーシック(レーザー上皮内角膜炎)
- 屈折矯正角膜切除術(PRK)
- 有水晶体眼内レンズ(IOL)
- 乱視角膜切開術(AK)
- 自動ラメラ角膜移植術(ALK)
- 角膜内リング(INTACS)
- レーザー熱角膜移植術 (LTK)
- 導電性角膜移植(CK)
- 放射状角膜切開術(RK)
- その他
手術の種類に基づいて、屈折矯正手術装置市場は、LASIK(レーザー角膜内切削術)、光屈折角膜切除術(PRK)、有水晶体眼内レンズ(IOL)、乱視角膜切開術(AK)、自動ラメラ角膜移植術(ALK)、角膜内リング(INTACS)、レーザー熱角膜移植術(LTK)、導電性角膜移植術(CK)、放射状角膜切開術(RK)などに分類されます。
応用
- 近視
- 遠視
- 乱視
- 老眼
用途に基づいて、屈折矯正手術装置市場は、近視、遠視、乱視、老眼に分類されます。
エンドユーザー
- 病院
- 専門クリニック
- 外来手術センター
- その他
エンドユーザーに基づいて、屈折矯正手術装置市場は、病院、専門クリニック、外来手術センター、その他に分類されます。
流通チャネル
- 直接入札
- サードパーティ販売業者
- その他

On the basis of distribution channel, the refractive surgery devices market is segmented into direct tender, third party distributors, and others.
Refractive Surgery Devices Market Regional Analysis/Insights
The refractive surgery devices market is analysed and market size insights and trends are provided by country, product type, surgery type, application, end user, and distribution channel as referenced above.
The countries covered in the refractive surgery devices market report are the Germany, France, U.K., Italy, Spain, Netherlands, Russia Switzerland, Belgium, Turkey, Austria, Norway, Hungary, Lithuania, Ireland, Poland Rest of Europe.
Germany is expected to dominate the market due to the rising adoption of minimal invasive surgeries.
The country section of the report also provides individual market impacting factors and changes in regulations in the market that impact the current and future trends of the market. Data points, such as new and replacement sales, country demographics, disease epidemiology, and import-export tariffs, are some of the major pointers used to forecast the market scenario for individual countries. In addition, the presence and availability of global brands and their challenges faced due to high competition from local and domestic brands, and impact of sales channels are considered while providing forecast analysis of the country data.
Competitive Landscape and Refractive Surgery Devices Market Share Analysis
The refractive surgery devices market competitive landscape provides details by the competitors. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, and application dominance. The above data points provided are only related to the companies' focus on refractive surgery devices market.
Some of the major companies dealing in the refractive surgery devices market are Tracey Technologies, Bausch + Lomb Incorporated, BD, STAAR SURGICAL, SCHWIND eye-tech-solutions, Hoya Surgical Optics, Johnson & Johnson Services, Inc., Ophtec BV, Glaukos Corporation, Amplitude Laser, Reichert, Inc., NIDEK CO., LTD., Ziemer Ophthalmic Systems, ROWIAK GmbH, Moria, LENSAR, Inc., Topcon Canada Inc. (A subsidiary of Topcon Corporation), Aaren Scientific Inc., Rayner Intraocular Lenses Limited., iVIS Technologies, Alcon, among others.
Research Methodology
データ収集と基準年分析は、大規模なサンプル サイズのデータ収集モジュールを使用して行われます。市場データは、市場統計モデルとコヒーレント モデルを使用して分析および推定されます。さらに、市場シェア分析と主要トレンド分析は、市場レポートの主要な成功要因です。DBMR 調査チームが使用する主要な調査方法は、データ マイニング、データ変数の市場への影響の分析、および一次 (業界の専門家) 検証を含むデータ三角測量です。これとは別に、データ モデルには、ベンダー ポジショニング グリッド、市場タイムライン分析、市場概要とガイド、企業ポジショニング グリッド、企業市場シェア分析、測定基準、世界対地域、ベンダー シェア分析が含まれます。さらに問い合わせる場合は、アナリストへの電話をリクエストしてください。
カスタマイズ可能
Data Bridge Market Research は、高度な形成的調査のリーダーです。当社は、既存および新規のお客様に、お客様の目標に合ったデータと分析を提供することに誇りを持っています。レポートは、対象ブランドの価格動向分析、追加国の市場理解 (国のリストをお問い合わせください)、臨床試験結果データ、文献レビュー、リファービッシュ市場、製品ベース分析などを含めるようにカスタマイズできます。対象競合他社の市場分析は、技術ベースの分析から市場ポートフォリオ戦略まで分析できます。必要な競合他社を必要なだけ追加できます。必要な形式とデータ スタイルでデータを追加できます。当社のアナリスト チームは、粗い生の Excel ファイル ピボット テーブル (ファクトブック) でデータを提供したり、レポートで利用可能なデータ セットからプレゼンテーションを作成するお手伝いをしたりすることもできます。
SKU-
世界初のマーケットインテリジェンスクラウドに関するレポートにオンラインでアクセスする
- インタラクティブなデータ分析ダッシュボード
- 成長の可能性が高い機会のための企業分析ダッシュボード
- カスタマイズとクエリのためのリサーチアナリストアクセス
- インタラクティブなダッシュボードによる競合分析
- 最新ニュース、更新情報、トレンド分析
- 包括的な競合追跡のためのベンチマーク分析のパワーを活用
目次
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF EUROPE REFRACTIVE SURGERY DEVICES MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 PRODUCT TYPE LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET APPLICATION COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL'S MODEL
4.2 PORTER'S FIVE FORCES MODEL
4.3 EUROPE REFRACTIVE SURGERY DEVICES MARKET: REGULATIONS
4.3.1 REGULATION IN THE U.S.
4.3.2 REGULATIONS IN EUROPE
4.3.3 REGULATIONS IN SINGAPORE
4.3.4 REGULATIONS IN AUSTRALIA
5 MARKET OVERVIEW
5.1 DRIVERS
5.1.1 INCREASING TECHNOLOGICAL ADVANCEMENT
5.1.2 RISE IN HEALTHCARE EXPENDITURE
5.1.3 INCREASE IN POPULATION WITH MACULAR DEGENERATION
5.1.4 RISE IN ADOPTION OF MINIMALLY INVASIVE SURGERIES
5.2 RESTRAINTS
5.2.1 STRINGENT RULES AND REGULATIONS
5.2.2 HIGH COST ASSOCIATED WITH REFRACTIVE SURGERY DEVICES
5.2.3 SIDE EFFECTS OF SURGERY
5.3 OPPORTUNITIES
5.3.1 ACHIEVEMENTS IN LASIK SURGERIES
5.3.2 STRATEGIC INITIATIVES BY MARKET PLAYERS
5.3.3 INCREASING GERIATRIC POPULATION
5.3.4 EXCESSIVE USAGE OF DIGITAL DEVICES
5.4 CHALLENGES
5.4.1 DEARTH OF SKILLED PROFESSIONALS
5.4.2 LACK OF HEALTHCARE FACILITIES FOR EYE TREATMENT
6 COVID-19 IMPACT ON EUROPE REFRACTIVE SURGERY DEVICES MARKET
6.1 IMPACT ON PRICE
6.2 IMPACT ON DEMAND
6.3 IMPACT ON SUPPLY
6.4 STRATEGIC DECISIONS BY MANUFACTURERS
6.5 CONCLUSION
7 EUROPE REFRACTIVE SURGERY DEVICES MARKET, BY PRODUCT TYPE
7.1 OVERVIEW
7.2 LASER
7.2.1 EXCIMER LASERS
7.2.2 FEMTOSECOND LASER/ULTRASHORT PULSE LASER
7.2.3 OTHERS
7.3 PHAKIC INTRAOCULAR LENS (IOL)
7.4 ABERROMETERS / WAVEFRONT ABERROMETRY
7.5 SURGICAL INSTRUMENTS & ACCESSORIES
7.6 REFRACTIVE SURGERY KITS
7.7 PUPILLARY DIAMETER METERS
7.8 EPIKERATOMES
7.9 MICROKERATOMES
7.1 THERMOKERATOPLASTY
7.11 LIMBAL RELAXING INCISION KITS
7.12 OTHERS
8 EUROPE REFRACTIVE SURGERY DEVICES DISEASE MARKET, BY SURGERY TYPE
8.1 OVERVIEW
8.2 LASIK (LASER IN-SITU KERATOMILEUSIS)
8.3 PHOTOREFRACTIVE KERATECTOMY (PRK)
8.4 PHAKIC INTRAOCULAR LENSES (IOL)
8.5 ASTIGMATIC KERATOTOMY (AK)
8.6 AUTOMATED LAMELLAR KERATOPLASTY (ALK)
8.7 INTRACORNEAL RING (INTACS)
8.8 LASER THERMAL KERATOPLASTY (LTK)
8.9 CONDUCTIVE KERATOPLASTY (CK)
8.1 RADIAL KERATOTOMY (RK)
8.11 OTHERS
9 EUROPE REFRACTIVE SURGERY DEVICES DISEASE MARKET, BY APPLICATION
9.1 OVERVIEW
9.2 NEARSIGHTEDNESS (MYOPIA)
9.3 FARSIGHTEDNESS (HYPEROPIA)
9.4 PRESBYOPIA
9.5 ASTIGMATISM
10 EUROPE REFRACTIVE SURGERY DEVICES DISEASE MARKET, BY END USER
10.1 OVERVIEW
10.2 HOSPITAL
10.3 SPECIALTY CLINICS
10.4 AMBULATORY SURGICAL CENTERS
10.5 OTHERS
11 EUROPE REFRACTIVE SURGERY DEVICES DISEASE MARKET, BY DISTRIBUTION CHANNEL
11.1 OVERVIEW
11.2 DIRECT TENDER
11.3 THIRD PARTY DISTRIBUTORS
11.4 OTHERS
12 EUROPE REFRACTIVE SURGERY DEVICES MARKET, BY REGION
12.1 EUROPE
12.1.1 GERMANY
12.1.2 FRANCE
12.1.3 U.K.
12.1.4 ITALY
12.1.5 SPAIN
12.1.6 NETHERLANDS
12.1.7 RUSSIA
12.1.8 SWITZERLAND
12.1.9 BELGIUM
12.1.10 TURKEY
12.1.11 AUSTRIA
12.1.12 NORWAY
12.1.13 HUNGARY
12.1.14 LITHUANIA
12.1.15 IRELAND
12.1.16 POLAND
12.1.17 REST OF EUROPE
13 EUROPE REFRACTIVE SURGERY DEVICES MARKET: COMPANY LANDSCAPE
13.1 COMPANY SHARE ANALYSIS: EUROPE
14 SWOT ANALYSIS
15 COMPANY PROFILE
15.1 JOHNSON AND JOHNSON SERVICES, INC.
15.1.1 COMPANY SNAPSHOT
15.1.2 REVENUE ANALYSIS
15.1.3 COMPANY SHARE ANALYSIS
15.1.4 PRODUCT PORTFOLIO
15.1.5 RECENT DEVELOPMENT
15.1.5.1 PRODUCT LAUNCH
15.2 ALCON INC.
15.2.1 COMPANY SNAPSHOT
15.2.2 REVENUE ANALYSIS
15.2.3 COMPANY SHARE ANALYSIS
15.2.4 PRODUCT PORTFOLIO
15.2.5 RECENT DEVELOPMENTS
15.2.5.1 ACQUISITION
15.2.5.2 PRODUCT LAUNCH
15.3 STAAR SURGICAL
15.3.1 COMPANY SNAPSHOT
15.3.2 REVENUE ANALYSIS
15.3.3 COMPANY SHARE ANALYSIS
15.3.4 PRODUCT PORTFOLIO
15.3.5 RECENT DEVELOPMENTS
15.4 BAUSCH + LOMB INCORPORATED
15.4.1 COMPANY SNAPSHOT
15.4.2 REVENUE ANALYSIS
15.4.3 COMPANY SHARE ANALYSIS
15.4.4 PRODUCT PORTFOLIO
15.4.5 RECENT DEVELOPMENT
15.4.5.1 ACQUISITION
15.4.5.2 CE APPROVAL
15.5 TOPCON CANADA INC., (A SUBSIDIARY OF TOPCON CORPORATION)
15.5.1 COMPANY SNAPSHOT
15.5.2 REVENUE ANALYSIS
15.5.3 COMPANY SHARE ANALYSIS
15.5.4 PRODUCT PORTFOLIO
15.5.5 RECENT DEVELOPMENTS
15.5.5.1 PARTNERSHIP
15.5.5.2 ACQUISITION
15.6 AAREN SCIENTIFIC INC.
15.6.1 COMPANY SNAPSHOT
15.6.2 PRODUCT PORTFOLIO
15.6.3 RECENT DEVELOPMENT
15.7 AMPLITUDE LASER
15.7.1 COMPANY SNAPSHOT
15.7.2 PRODUCT PORTFOLIO
15.7.3 RECENT DEVELOPMENTS
15.7.3.1 PARTNERSHIP
15.8 BD
15.8.1 COMPANY SNAPSHOT
15.8.2 REVENUE ANALYSIS
15.8.3 PRODUCT PORTFOLIO
15.8.4 RECENT DEVELOPMENTS
15.8.4.1 CONFERENCE
15.8.4.2 PRODUCT LAUNCH
15.9 GLAUKOS CORPORATION
15.9.1 COMPANY SNAPSHOT
15.9.2 REVENUE ANALYSIS
15.9.3 PRODUCT PORTFOLIO
15.9.4 RECENT DEVELOPMENTS
15.9.4.1 PRODUCT LAUNCH
15.9.4.2 ACQUISITION
15.1 HOYA SURGICAL OPTICS
15.10.1 COMPANY SNAPSHOT
15.10.2 PRODUCT PORTFOLIO
15.10.3 RECENT DEVELOPMENT
15.10.3.1 CONFERENCE
15.11 IVIS TECHNOLOGIES
15.11.1 COMPANY SNAPSHOT
15.11.2 PRODUCT PORTFOLIO
15.11.3 RECENT DEVELOPMENTS
15.12 LENSAR INC. (A SUBSDIARY OF PDL BIOPHARMA, INC.)
15.12.1 COMPANY SNAPSHOT
15.12.2 PRODUCT PORTFOLIO
15.12.3 RECENT DEVELOPMENTS
15.13 MORIA
15.13.1 COMPANY SNAPSHOT
15.13.2 PRODUCT PORTFOLIO
15.13.3 RECENT DEVELOPMENT
15.14 NIDEK CO., LTD
15.14.1 COMPANY SNAPSHOT
15.14.2 PRODUCT PORTFOLIO
15.14.3 RECENT DEVELOPMENT
15.14.3.1 WEBSITE LAUNCH
15.15 OPHTEC BV
15.15.1 COMPANY SNAPSHOT
15.15.2 PRODUCT PORTFOLIO
15.15.3 RECENT DEVELOPMENT
15.15.3.1 PRODUCT LAUNCH
15.16 RAYNER INTRAOCULAR LENSES LIMITED
15.16.1 COMPANY SNAPSHOT
15.16.2 PRODUCT PORTFOLIO
15.16.3 RECENT DEVELOPMENTS
15.16.3.1 NEW DISTRIBUTION UNIT
15.16.3.2 ACQUISITION
15.16.3.3 ACQUISITION
15.17 REICHERT, INC.
15.17.1 COMPANY SNAPSHOT
15.17.2 PRODUCT PORTFOLIO
15.17.3 RECENT DEVELOPMENT
15.17.3.1 CONFERENCE
15.18 ROWIAK GMBH
15.18.1 COMPANY SNAPSHOT
15.18.2 PRODUCT PORTFOLIO
15.18.3 RECENT DEVELOPMENT
15.18.3.1 R&D FACILITY
15.19 SCHWIND EYE-TECH-SOLUTIONS
15.19.1 COMPANY SNAPSHOT
15.19.2 PRODUCT PORTFOLIO
15.19.3 RECENT DEVELOPMENTS
15.2 TRACEY TECHNOLOGIES
15.20.1 COMPANY SNAPSHOT
15.20.2 PRODUCT PORTFOLIO
15.20.3 RECENT DEVELOPMENT
15.20.3.1 R&D FACILITY
15.21 ZIEMER OPHTHALMIC SYSTEMS
15.21.1 COMPANY SNAPSHOT
15.21.2 PRODUCT PORTFOLIO
15.21.3 RECENT DEVELOPMENT
15.21.3.1 AGREEMENT
16 QUESTIONNAIRE
17 RELATED REPORTS
表のリスト
TABLE 1 EUROPE REFRACTIVE SURGERY DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 2 EUROPE LASER IN REFRACTIVE SURGERY DEVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 3 EUROPE LASER IN REFRACTIVE SURGERY DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 4 EUROPE PHAKIC INTRAOCULAR LENS (IOL) IN REFRACTIVE SURGERY DEIVCES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 5 EUROPE ABBEROMETERS/WAFEFRONT ABERROMETRY IN REFRACTIVE SURGERY DEVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 6 EUROPE SURGICAL INSTRUMENT & ACCESSORIES IN REFRACTIVE SURGERY DEVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 7 EUROPE REFRACTIVE SURGERY KITS IN REFRACTIVE SURGERY DEVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 8 EUROPE PUPILLARY DIAMETER METERS IN REFRACTIVE SURGERY DEVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 9 EUROPE EPIKERATOMES IN REFRACTIVE SURGERY DEIVCES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 10 EUROPE MICROKERATOMES IN REFRACTIVE SURGERY DEVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 11 EUROPE THERMOKERATOPLASTY IN REFRACTIVE SURGERY DEVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 12 EUROPE LIMBAL RELAXING INCISION KITS IN REFRACTIVE SURGERY DEVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 13 EUROPE OTHERS IN REFRACTIVE SURGERY DEVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 14 EUROPE REFRACTIVE SURGERY DEVICES MARKET, BY SURGERY TYPE, 2020-2029 (USD MILLION)
TABLE 15 EUROPE LASIK (LASER IN-SITU KERATOMILEUSIS) IN REFRACTIVE SURGERY DEVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 16 EUROPE PHOTOREFRACTIVE KERATECTOMY (PRK) IN REFRACTIVE SURGERY DEIVCES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 17 EUROPE PHAKIC INTRAOCULAR LENSES (IOL) IN REFRACTIVE SURGERY DEVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 18 EUROPE ASTIGMATIC KERATOTOMY (AK) IN REFRACTIVE SURGERY DEVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 19 EUROPE AUTOMATED LAMELLAR KERATOPLASTY (ALK) IN REFRACTIVE SURGERY DEVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 20 EUROPE INTRACORNEAL RING (INTACS) IN REFRACTIVE SURGERY DEVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 21 EUROPE LASER THERMAL KERATOPLASTY (LTK) IN REFRACTIVE SURGERY DEIVCES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 22 EUROPE CONDUCTIVE KERATOPLASTY (CK) IN REFRACTIVE SURGERY DEVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 23 EUROPE RADIAL KERATOTOMY (RK) IN REFRACTIVE SURGERY DEVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 24 EUROPE OTHERS IN REFRACTIVE SURGERY DEVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 25 EUROPE REFRACTIVE SURGERY DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 26 EUROPE NEARSIGHTEDNESS (MYOPIA) IN REFRACTIVE SURGERY DEVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 27 EUROPE FARSIGHTEDNESS (HYPEROPIA) IN REFRACTIVE SURGERY DEIVCES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 28 EUROPE PRESBYOPIA IN REFRACTIVE SURGERY DEVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 29 EUROPE ASTIGMATISM IN REFRACTIVE SURGERY DEVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 30 EUROPE REFRACTIVE SURGERY DEVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 31 EUROPE HOSPITALS IN REFRACTIVE SURGERY DEVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 32 EUROPE SPECIALTY CLINICS IN REFRACTIVE SURGERY DEIVCES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 33 EUROPE AMBULATORY SURGICAL CENTERS IN REFRACTIVE SURGERY DEVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 34 EUROPE OTHERS IN REFRACTIVE SURGERY DEVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 35 EUROPE REFRACTIVE SURGERY DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 36 EUROPE DIRECT TENDER IN REFRACTIVE SURGERY DEVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 37 EUROPE THIRD PARTY DISTRIBUTORS IN REFRACTIVE SURGERY DEIVCES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 38 EUROPE OTHERS IN REFRACTIVE SURGERY DEVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 39 EUROPE REFRACTIVE SURGERY DEVICES MARKET, BY COUNTRY, 2020-2029 (USD MILLION)
TABLE 40 EUROPE REFRACTIVE SURGERY DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 41 EUROPE LASER IN REFRACTIVE SURGERY DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 42 EUROPE REFRACTIVE SURGERY DEVICES MARKET, BY SURGERY TYPE, 2020-2029 (USD MILLION)
TABLE 43 EUROPE REFRACTIVE SURGERY DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 44 EUROPE REFRACTIVE SURGERY DEVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 45 EUROPE REFRACTIVE SURGERY DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 46 GERMANY REFRACTIVE SURGERY DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 47 GERMANY LASER IN REFRACTIVE SURGERY DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 48 GERMANY REFRACTIVE SURGERY DEVICES MARKET, BY SURGERY TYPE, 2020-2029 (USD MILLION)
TABLE 49 GERMANY REFRACTIVE SURGERY DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 50 GERMANY REFRACTIVE SURGERY DEVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 51 GERMANY REFRACTIVE SURGERY DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 52 FRANCE REFRACTIVE SURGERY DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 53 FRANCE LASER IN REFRACTIVE SURGERY DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 54 FRANCE REFRACTIVE SURGERY DEVICES MARKET, BY SURGERY TYPE, 2020-2029 (USD MILLION)
TABLE 55 FRANCE REFRACTIVE SURGERY DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 56 FRANCE REFRACTIVE SURGERY DEVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 57 FRANCE REFRACTIVE SURGERY DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 58 U.K. REFRACTIVE SURGERY DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 59 U.K. LASER IN REFRACTIVE SURGERY DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 60 U.K. REFRACTIVE SURGERY DEVICES MARKET, BY SURGERY TYPE, 2020-2029 (USD MILLION)
TABLE 61 U.K. REFRACTIVE SURGERY DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 62 U.K. REFRACTIVE SURGERY DEVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 63 U.K. REFRACTIVE SURGERY DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 64 ITALY REFRACTIVE SURGERY DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 65 ITALY LASER IN REFRACTIVE SURGERY DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 66 ITALY REFRACTIVE SURGERY DEVICES MARKET, BY SURGERY TYPE, 2020-2029 (USD MILLION)
TABLE 67 ITALY REFRACTIVE SURGERY DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 68 ITALY REFRACTIVE SURGERY DEVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 69 ITALY REFRACTIVE SURGERY DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 70 SPAIN REFRACTIVE SURGERY DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 71 SPAIN LASER IN REFRACTIVE SURGERY DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 72 SPAIN REFRACTIVE SURGERY DEVICES MARKET, BY SURGERY TYPE, 2020-2029 (USD MILLION)
TABLE 73 SPAIN REFRACTIVE SURGERY DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 74 SPAIN REFRACTIVE SURGERY DEVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 75 SPAIN REFRACTIVE SURGERY DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 76 NETHERLANDS REFRACTIVE SURGERY DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 77 NETHERLANDS LASER IN REFRACTIVE SURGERY DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 78 NETHERLANDS REFRACTIVE SURGERY DEVICES MARKET, BY SURGERY TYPE, 2020-2029 (USD MILLION)
TABLE 79 NETHERLANDS REFRACTIVE SURGERY DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 80 NETHERLANDS REFRACTIVE SURGERY DEVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 81 NETHERLANDS REFRACTIVE SURGERY DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 82 RUSSIA REFRACTIVE SURGERY DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 83 RUSSIA LASER IN REFRACTIVE SURGERY DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 84 RUSSIA REFRACTIVE SURGERY DEVICES MARKET, BY SURGERY TYPE, 2020-2029 (USD MILLION)
TABLE 85 RUSSIA REFRACTIVE SURGERY DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 86 RUSSIA REFRACTIVE SURGERY DEVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 87 RUSSIA REFRACTIVE SURGERY DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 88 SWITZERLAND REFRACTIVE SURGERY DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 89 SWITZERLAND LASER IN REFRACTIVE SURGERY DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 90 SWITZERLAND REFRACTIVE SURGERY DEVICES MARKET, BY SURGERY TYPE, 2020-2029 (USD MILLION)
TABLE 91 SWITZERLAND REFRACTIVE SURGERY DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 92 SWITZERLAND REFRACTIVE SURGERY DEVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 93 SWITZERLAND REFRACTIVE SURGERY DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 94 BELGIUM REFRACTIVE SURGERY DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 95 BELGIUM LASER IN REFRACTIVE SURGERY DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 96 BELGIUM REFRACTIVE SURGERY DEVICES MARKET, BY SURGERY TYPE, 2020-2029 (USD MILLION)
TABLE 97 BELGIUM REFRACTIVE SURGERY DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 98 BELGIUM REFRACTIVE SURGERY DEVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 99 BELGIUM REFRACTIVE SURGERY DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 100 TURKEY REFRACTIVE SURGERY DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 101 TURKEY LASER IN REFRACTIVE SURGERY DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 102 TURKEY REFRACTIVE SURGERY DEVICES MARKET, BY SURGERY TYPE, 2020-2029 (USD MILLION)
TABLE 103 TURKEY REFRACTIVE SURGERY DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 104 TURKEY REFRACTIVE SURGERY DEVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 105 TURKEY REFRACTIVE SURGERY DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 106 AUSTRIA REFRACTIVE SURGERY DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 107 AUSTRIA LASER IN REFRACTIVE SURGERY DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 108 AUSTRIA REFRACTIVE SURGERY DEVICES MARKET, BY SURGERY TYPE, 2020-2029 (USD MILLION)
TABLE 109 AUSTRIA REFRACTIVE SURGERY DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 110 AUSTRIA REFRACTIVE SURGERY DEVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 111 AUSTRIA REFRACTIVE SURGERY DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 112 NORWAY REFRACTIVE SURGERY DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 113 NORWAY LASER IN REFRACTIVE SURGERY DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 114 NORWAY REFRACTIVE SURGERY DEVICES MARKET, BY SURGERY TYPE, 2020-2029 (USD MILLION)
TABLE 115 NORWAY REFRACTIVE SURGERY DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 116 NORWAY REFRACTIVE SURGERY DEVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 117 NORWAY REFRACTIVE SURGERY DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 118 HUNGARY REFRACTIVE SURGERY DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 119 HUNGARY LASER IN REFRACTIVE SURGERY DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 120 HUNGARY REFRACTIVE SURGERY DEVICES MARKET, BY SURGERY TYPE, 2020-2029 (USD MILLION)
TABLE 121 HUNGARY REFRACTIVE SURGERY DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 122 HUNGARY REFRACTIVE SURGERY DEVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 123 HUNGARY REFRACTIVE SURGERY DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 124 LITHUANIA REFRACTIVE SURGERY DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 125 LITHUANIA LASER IN REFRACTIVE SURGERY DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 126 LITHUANIA REFRACTIVE SURGERY DEVICES MARKET, BY SURGERY TYPE, 2020-2029 (USD MILLION)
TABLE 127 LITHUANIA REFRACTIVE SURGERY DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 128 LITHUANIA REFRACTIVE SURGERY DEVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 129 LITHUANIA REFRACTIVE SURGERY DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 130 IRELAND REFRACTIVE SURGERY DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 131 IRELAND LASER IN REFRACTIVE SURGERY DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 132 IRELAND REFRACTIVE SURGERY DEVICES MARKET, BY SURGERY TYPE, 2020-2029 (USD MILLION)
TABLE 133 IRELAND REFRACTIVE SURGERY DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 134 IRELAND REFRACTIVE SURGERY DEVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 135 IRELAND REFRACTIVE SURGERY DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 136 POLAND REFRACTIVE SURGERY DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 137 POLAND LASER IN REFRACTIVE SURGERY DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 138 POLAND REFRACTIVE SURGERY DEVICES MARKET, BY SURGERY TYPE, 2020-2029 (USD MILLION)
TABLE 139 POLAND REFRACTIVE SURGERY DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 140 POLAND REFRACTIVE SURGERY DEVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 141 POLAND REFRACTIVE SURGERY DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 142 REST OF EUROPE REFRACTIVE SURGERY DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
図表一覧
FIGURE 1 EUROPE REFRACTIVE SURGERY DEVICES MARKET: SEGMENTATION
FIGURE 2 EUROPE REFRACTIVE SURGERY DEVICES MARKET: DATA TRIANGULATION
FIGURE 3 EUROPE REFRACTIVE SURGERY DEVICES MARKET: DROC ANALYSIS
FIGURE 4 EUROPE REFRACTIVE SURGERY DEVICES MARKET: EUROPE VS REGIONAL MARKET ANALYSIS
FIGURE 5 EUROPE REFRACTIVE SURGERY DEVICES MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 EUROPE REFRACTIVE SURGERY DEVICES MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 EUROPE REFRACTIVE SURGERY DEVICES MARKET: DBMR MARKET POSITION GRID
FIGURE 8 EUROPE REFRACTIVE SURGERY DEVICES MARKET: MARKET APPLICATION COVERAGE GRID
FIGURE 9 EUROPE REFRACTIVE SURGERY DEVICES MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 EUROPE REFRACTIVE SURGERY DEVICES MARKET: SEGMENTATION
FIGURE 11 INCREASING TECHNOLOGICAL ADVANCEMENTS IN THE REFRACTIVE SURGERY DEVICES ARE EXPECTED TO DRIVE THE EUROPE REFRACTIVE SURGERY DEVICES MARKET IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 12 PRODUCT TYPE SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE EUROPE REFRACTIVE SURGERY DEVICES MARKET IN 2022 & 2029
FIGURE 13 NORTH AMERICA IS EXPECTED TO DOMINATE THE EUROPE REFRACTIVE SURGERY DEVICES MARKET, AND ASIA-PACIFIC IS EXPECTED TO GROW WITH THE HIGHEST CAGR IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 14 DRIVERS, RESTRAINT, OPPORTUNITIES, CHALLENGES FOR EUROPE REFRACTIVE SURGERY DEVICES MARKET
FIGURE 15 EUROPE REFRACTIVE SURGERY DEVICES DISEASE MARKET: BY PRODUCT TYPE, 2021
FIGURE 16 EUROPE REFRACTIVE SURGERY DEVICES DISEASE MARKET: BY PRODUCT TYPE, 2020-2029 (USD MILLION)
FIGURE 17 EUROPE REFRACTIVE SURGERY DEVICES DISEASE MARKET: BY PRODUCT TYPE, CAGR (2022-2029)
FIGURE 18 EUROPE REFRACTIVE SURGERY DEVICES DISEASE MARKET: BY PRODUCT TYPE, LIFELINE CURVE
FIGURE 19 EUROPE REFRACTIVE SURGERY DEVICES DISEASE MARKET: BY SURGERY TYPE, 2021
FIGURE 20 EUROPE REFRACTIVE SURGERY DEVICES DISEASE MARKET: BY SURGERY TYPE, 2020-2029 (USD MILLION)
FIGURE 21 EUROPE REFRACTIVE SURGERY DEVICES DISEASE MARKET: BY SURGERY TYPE, CAGR (2022-2029)
FIGURE 22 EUROPE REFRACTIVE SURGERY DEVICES DISEASE MARKET: BY SURGERY TYPE, LIFELINE CURVE
FIGURE 23 EUROPE REFRACTIVE SURGERY DEVICES DISEASE MARKET: BY APPLICATION, 2021
FIGURE 24 EUROPE REFRACTIVE SURGERY DEVICES DISEASE MARKET: BY APPLICATION, 2020-2029 (USD MILLION)
FIGURE 25 EUROPE REFRACTIVE SURGERY DEVICES DISEASE MARKET: BY APPLICATION, CAGR (2022-2029)
FIGURE 26 EUROPE REFRACTIVE SURGERY DEVICES DISEASE MARKET: BY APPLICATION, LIFELINE CURVE
FIGURE 27 EUROPE REFRACTIVE SURGERY DEVICES DISEASE MARKET: BY END USER, 2021
FIGURE 28 EUROPE REFRACTIVE SURGERY DEVICES DISEASE MARKET: BY END USER, 2020-2029 (USD MILLION)
FIGURE 29 EUROPE REFRACTIVE SURGERY DEVICES DISEASE MARKET: BY END USER, CAGR (2022-2029)
FIGURE 30 EUROPE REFRACTIVE SURGERY DEVICES DISEASE MARKET: BY END USER, LIFELINE CURVE
FIGURE 31 EUROPE REFRACTIVE SURGERY DEVICES DISEASE MARKET: BY DISTRIBUTION CHANNEL, 2021
FIGURE 32 EUROPE REFRACTIVE SURGERY DEVICES DISEASE MARKET: BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
FIGURE 33 EUROPE REFRACTIVE SURGERY DEVICES DISEASE MARKET: BY DISTRIBUTION CHANNEL, CAGR (2022-2029)
FIGURE 34 EUROPE REFRACTIVE SURGERY DEVICES DISEASE MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 35 EUROPE REFRACTIVE SURGERY DEVICES MARKET: SNAPSHOT (2021)
FIGURE 36 EUROPE REFRACTIVE SURGERY DEVICES MARKET: BY COUNTRY (2021)
FIGURE 37 EUROPE REFRACTIVE SURGERY DEVICES MARKET: BY COUNTRY (2022 & 2029)
FIGURE 38 EUROPE REFRACTIVE SURGERY DEVICES MARKET: BY COUNTRY (2021 & 2029)
FIGURE 39 EUROPE REFRACTIVE SURGERY DEVICES MARKET: BY PRODUCT TYPE (2022-2029)
FIGURE 40 EUROPE REFRACTIVE SURGERY DEVICES MARKET: COMPANY SHARE 2021 (%)

調査方法
データ収集と基準年分析は、大規模なサンプル サイズのデータ収集モジュールを使用して行われます。この段階では、さまざまなソースと戦略を通じて市場情報または関連データを取得します。過去に取得したすべてのデータを事前に調査および計画することも含まれます。また、さまざまな情報ソース間で見られる情報の不一致の調査も含まれます。市場データは、市場統計モデルと一貫性モデルを使用して分析および推定されます。また、市場シェア分析と主要トレンド分析は、市場レポートの主要な成功要因です。詳細については、アナリストへの電話をリクエストするか、お問い合わせをドロップダウンしてください。
DBMR 調査チームが使用する主要な調査方法は、データ マイニング、データ変数が市場に与える影響の分析、および一次 (業界の専門家) 検証を含むデータ三角測量です。データ モデルには、ベンダー ポジショニング グリッド、市場タイムライン分析、市場概要とガイド、企業ポジショニング グリッド、特許分析、価格分析、企業市場シェア分析、測定基準、グローバルと地域、ベンダー シェア分析が含まれます。調査方法について詳しくは、お問い合わせフォームから当社の業界専門家にご相談ください。
カスタマイズ可能
Data Bridge Market Research は、高度な形成的調査のリーダーです。当社は、既存および新規のお客様に、お客様の目標に合致し、それに適したデータと分析を提供することに誇りを持っています。レポートは、対象ブランドの価格動向分析、追加国の市場理解 (国のリストをお問い合わせください)、臨床試験結果データ、文献レビュー、リファービッシュ市場および製品ベース分析を含めるようにカスタマイズできます。対象競合他社の市場分析は、技術ベースの分析から市場ポートフォリオ戦略まで分析できます。必要な競合他社のデータを、必要な形式とデータ スタイルでいくつでも追加できます。当社のアナリスト チームは、粗い生の Excel ファイル ピボット テーブル (ファクト ブック) でデータを提供したり、レポートで利用可能なデータ セットからプレゼンテーションを作成するお手伝いをしたりすることもできます。