Global Cell Culture Media and Single
Market Size in USD Billion
CAGR :
% 
 USD
4.11 Billion
USD
8.24 Billion
2025
2033
USD
4.11 Billion
USD
8.24 Billion
2025
2033
| 2026 –2033 | |
| USD 4.11 Billion | |
| USD 8.24 Billion | |
|
|
|
|
Global Cell Culture Media & Single-Use Reagents Market Segmentation, By Product Type (Cell Culture Media and Single-Use Reagents), Cell Type (Mammalian Cells, Stem Cells, Insect Cells, Microbial Cells, and Plant Cells), Application (Biopharmaceutical Production, Drug Screening & Development, Regenerative Medicine & Tissue Engineering, Stem Cell Technologies, and Cancer Research & Other Biotech R&D), End User (Pharmaceutical & Biotechnology Companies, Academic & Government Research Institutes, Contract Research Organizations, Hospitals & Diagnostic Laboratories, and Others)- Industry Trends and Forecast to 2033
Cell Culture Media & Single-Use Reagents Market Size
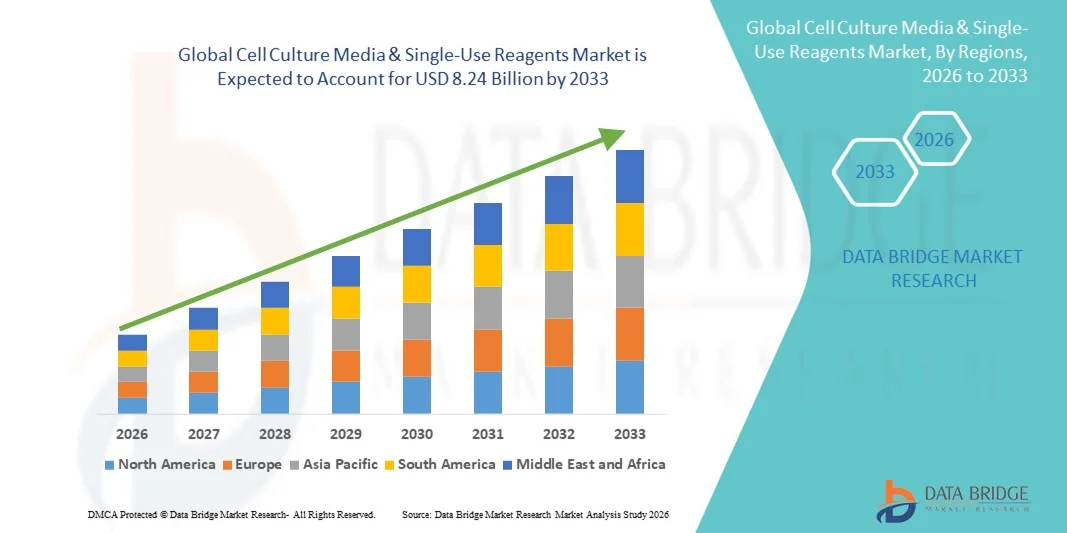
- The global cell culture media & single-use reagents market size was valued at USD 4.11 billion in 2025 and is expected to reach USD 8.24 billion by 2033, at a CAGR of 9.10% during the forecast period
- The market growth is largely driven by the expanding biopharmaceutical industry, increasing adoption of cell-based research, and rising demand for biologics, vaccines, and advanced therapies, which are accelerating the use of high-quality cell culture media and single-use reagents
- Furthermore, growing emphasis on contamination control, process efficiency, and regulatory compliance, along with the shift toward single-use and ready-to-use solutions in research and manufacturing environments, is positioning cell culture media and single-use reagents as essential components of modern life science workflows, thereby significantly boosting the industry’s growth
Cell Culture Media & Single-Use Reagents Market Analysis
- Cell culture media and single-use reagents, which provide essential nutrients, growth factors, and controlled environments for in-vitro cell growth, are increasingly critical components of modern life sciences, biopharmaceutical manufacturing, and clinical research workflows due to their role in ensuring reproducibility, scalability, and contamination-free processes
- The rising demand for cell culture media & single-use reagents is primarily driven by the rapid growth of the biopharmaceutical industry, increasing investments in biologics, vaccines, and cell- and gene-based therapies, along with expanding applications in drug discovery and regenerative medicine
- North America dominated the market with the largest revenue share of 38.5% in 2025, supported by a well-established biopharmaceutical sector, strong presence of leading life science companies, high R&D spending, and widespread adoption of advanced cell culture technologies, with the U.S. accounting for a major portion of demand due to its robust biologics pipeline and clinical research activity
- Asia-Pacific is expected to be the fastest-growing region during the forecast period owing to increasing pharmaceutical manufacturing capacity, growing investments in biotechnology research, expanding CRO/CDMO activities, and supportive government initiatives
- Single-use reagents segment dominated the market with market share of 45.7% in 2025, driven by their advantages in reducing cross-contamination risks, improving operational efficiency, and supporting flexible, scalable manufacturing processes in both research laboratories and large-scale bioproduction facilities
Report Scope and Cell Culture Media & Single-Use Reagents Market Segmentation
|
Attributes |
Cell Culture Media & Single-Use Reagents Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework |
Cell Culture Media & Single-Use Reagents Market Trends
Shift Toward Chemically Defined and Single-Use, Ready-to-Use Solutions
- A significant and accelerating trend in the global cell culture media & single-use reagents market is the transition toward chemically defined, serum-free, and ready-to-use single-use formulations, driven by the need for higher reproducibility, scalability, and regulatory compliance in biopharmaceutical manufacturing and advanced research workflows
- For instance, leading suppliers such as Thermo Fisher Scientific and Merck have expanded their portfolios of chemically defined media and pre-validated single-use reagents designed specifically for monoclonal antibody production and cell & gene therapy applications, reducing batch-to-batch variability
- The adoption of single-use reagents enables manufacturers to minimize contamination risks, shorten changeover times, and eliminate complex cleaning validation steps, which is particularly critical in multi-product biologics facilities and personalized medicine production
- Additionally, ready-to-use and pre-mixed media formats are gaining traction as they reduce preparation errors and labor requirements, allowing researchers and manufacturers to focus on process optimization rather than reagent handling
- This trend toward standardized, flexible, and high-performance media and single-use reagents is reshaping bioprocessing strategies, with companies increasingly prioritizing modular, disposable solutions to support rapid scale-up and evolving therapeutic pipelines
- Consequently, demand for advanced single-use reagents and optimized culture media continues to rise across biopharmaceutical companies, CROs, and CDMOs seeking efficiency, compliance, and faster time-to-market
Cell Culture Media & Single-Use Reagents Market Dynamics
Driver
Rising Biopharmaceutical Production and Growth of Cell-Based Therapies
- The rapid expansion of the biopharmaceutical industry, along with increasing development of biologics, vaccines, and cell- and gene-based therapies, is a major driver fueling demand for cell culture media and single-use reagents
- For instance, ongoing investments by pharmaceutical companies and CDMOs in biologics manufacturing capacity are increasing the need for high-quality, GMP-grade media and reagents that support consistent large-scale cell culture operations
- As complex biologics require precise control over cell growth and productivity, manufacturers rely heavily on optimized media formulations and single-use reagents to ensure product quality and process reliability
- Furthermore, the growth of clinical research activities and drug discovery programs is driving steady consumption of research-grade media and reagents across academic institutes and biotechnology companies
- Expanding investment in biologics manufacturing infrastructure, particularly large-scale bioreactor facilities and modular production units, is accelerating consumption of cell culture media and single-use reagents
- Increasing number of clinical trials involving monoclonal antibodies, vaccines, and cell-based therapies is boosting recurring demand for research- and GMP-grade media and reagents across development stages
- The need for scalable, flexible, and contamination-free production platforms is positioning cell culture media and single-use reagents as indispensable components of modern bioprocessing and life science research
Restraint/Challenge
High Cost, Supply Chain Dependence, and Regulatory Complexity
- The high cost of specialized cell culture media and single-use reagents, particularly chemically defined and GMP-grade products, presents a key challenge for small biotechnology firms and research institutions with limited budgets
- Additionally, heavy dependence on a limited number of global suppliers for critical raw materials and single-use components exposes manufacturers to supply chain disruptions, which can delay production and research timelines
- Strict regulatory requirements related to quality control, traceability, and validation of media and reagents further increase development and compliance costs for suppliers and end users
- Limited availability of critical raw materials used in specialized media and single-use reagents can lead to procurement challenges, longer lead times, and production delays for end users
- Disposal concerns related to plastic waste from single-use products and tightening environmental regulations may pose additional operational and compliance challenges for manufacturers and biopharmaceutical companies
- Variability in regulatory expectations across regions can complicate global product launches and standardization efforts, particularly for companies operating across multiple markets
- Addressing these challenges through cost optimization, supply chain diversification, and closer collaboration between suppliers and end users will be essential to ensure sustained growth and wider adoption of cell culture media and single-use reagents worldwide
Cell Culture Media & Single-Use Reagents Market Scope
The market is segmented on the basis of product type, cell type, application, and end user.
- By Product Type
On the basis of product type, the global cell culture media & single-use reagents market is segmented into cell culture media and single-use reagents. The single-use reagents segment dominated the market with a revenue share of 45.7% in 2025, driven by their widespread adoption in biopharmaceutical manufacturing and advanced research workflows. Single-use reagents reduce cross-contamination risks, eliminate cleaning and sterilization validation steps, and support closed and flexible processing environments. Their growing use in biologics, vaccines, and cell & gene therapy production has significantly increased demand. Additionally, regulatory agencies favor single-use systems due to improved traceability and process consistency. The shift toward modular and multi-product facilities further strengthens this segment’s dominance.
The cell culture media segment is expected to witness the fastest growth during the forecast period, fueled by increasing demand for chemically defined, serum-free, and specialty media. Rising development of biologics, biosimilars, and personalized therapies requires highly optimized media formulations to enhance cell productivity and product quality. Continuous innovation in media tailored for specific cell lines, along with growing consumption across research and clinical stages, is accelerating growth. Expanding biopharmaceutical pipelines globally further reinforce this trend.
- By Cell Type
On the basis of cell type, the market is segmented into mammalian cells, stem cells, insect cells, microbial cells, and plant cells. The mammalian cells segment dominated the market in 2025, owing to their extensive use in the production of monoclonal antibodies, recombinant proteins, vaccines, and other complex biologics. Mammalian cell lines such as CHO and HEK are widely preferred due to their ability to perform post-translational modifications similar to human cells. The strong pipeline of biologics and biosimilars, combined with increasing investments in large-scale mammalian cell culture facilities, continues to drive this segment’s leadership.
The stem cells segment is projected to grow at the fastest rate during the forecast period, driven by expanding research and commercialization of regenerative medicine, cell therapy, and personalized medicine applications. Increasing clinical trials involving stem cell-based therapies are boosting demand for specialized, high-quality culture media and single-use reagents. Moreover, advancements in induced pluripotent stem cell (iPSC) technologies and growing funding for stem cell research globally are further accelerating growth in this segment.
- By Application
On the basis of application, the market is segmented into biopharmaceutical production, drug screening & development, regenerative medicine & tissue engineering, stem cell technologies, and cancer research & other biotech R&D. The biopharmaceutical production segment held the largest market share in 2025, driven by the increasing global demand for biologics, vaccines, and biosimilars. Large-scale production of monoclonal antibodies and therapeutic proteins relies heavily on optimized cell culture media and single-use reagents to ensure high yields and consistent quality. The expansion of biologics manufacturing capacity and growing outsourcing to CDMOs further reinforce this segment’s dominance.
The regenerative medicine & tissue engineering segment is expected to witness the fastest growth during the forecast period, supported by rising interest in advanced therapies for chronic and degenerative diseases. These applications require highly specialized media and reagents to support cell differentiation, proliferation, and tissue formation. Increased funding, regulatory approvals for cell-based therapies, and technological advancements in tissue engineering are significantly accelerating demand in this segment.
- By End User
On the basis of end user, the market is segmented into pharmaceutical & biotechnology companies, academic & government research institutes, contract research organizations, hospitals & diagnostic laboratories, and others. The pharmaceutical & biotechnology companies segment dominated the market in 2025, owing to their extensive involvement in drug discovery, biologics development, and commercial manufacturing. These organizations consume large volumes of GMP-grade media and single-use reagents across research, clinical, and production stages. Continuous pipeline expansion and strong R&D investments further contribute to the segment’s leading position.
The contract research organizations (CROs) segment is anticipated to register the fastest growth during the forecast period, driven by increasing outsourcing of research, development, and manufacturing activities by pharmaceutical and biotechnology firms. CROs require standardized, ready-to-use media and single-use reagents to support diverse client projects while maintaining regulatory compliance and operational efficiency. The rising role of CROs in biologics development and clinical research is significantly boosting demand in this segment.
Cell Culture Media & Single-Use Reagents Market Regional Analysis
- North America dominated the market with the largest revenue share of 38.5% in 2025, supported by a well-established biopharmaceutical sector, strong presence of leading life science companies, high R&D spending, and widespread adoption of advanced cell culture technologies
- End users in the region place strong emphasis on high-quality, GMP-grade media and single-use reagents that ensure process consistency, regulatory compliance, and scalability across research and commercial manufacturing operations
- This widespread adoption is further supported by substantial healthcare and life sciences funding, a well-established research infrastructure, and the growing demand for biologics, vaccines, and cell- and gene-based therapies, positioning North America as a leading market for cell culture media and single-use reagents in both research and industrial applications
U.S. Cell Culture Media & Single-Use Reagents Market Insight
The U.S. cell culture media & single-use reagents market captured the largest revenue share within North America in 2025, driven by the country’s robust biopharmaceutical industry and extensive R&D ecosystem. The U.S. hosts a high concentration of leading biotechnology and pharmaceutical companies actively engaged in biologics, vaccines, and cell & gene therapy development. Strong funding for life sciences research, coupled with a large pipeline of clinical trials, continues to drive sustained demand for high-quality media and single-use reagents. Additionally, the presence of major suppliers and advanced manufacturing infrastructure supports rapid adoption of innovative and GMP-compliant products, reinforcing the U.S. market’s leadership position.
Europe Cell Culture Media & Single-Use Reagents Market Insight
The Europe cell culture media & single-use reagents market is projected to expand at a substantial CAGR during the forecast period, primarily driven by growing biologics production and stringent regulatory standards for drug development and manufacturing. Increasing investments in biotechnology research and rising demand for advanced therapies are fostering adoption across the region. European pharmaceutical companies and research institutes emphasize high-quality, standardized media and reagents to ensure compliance with strict quality and safety regulations. The market is witnessing steady growth across biopharmaceutical manufacturing, academic research, and CRO activities, particularly in Western European countries.
U.K. Cell Culture Media & Single-Use Reagents Market Insight
The U.K. cell culture media & single-use reagents market is anticipated to grow at a noteworthy CAGR during the forecast period, supported by strong government backing for life sciences and biotechnology innovation. The country’s active role in biologics research, vaccine development, and clinical trials is driving consistent demand for advanced culture media and reagents. The presence of leading academic institutions and research organizations further accelerates consumption. Additionally, the U.K.’s focus on precision medicine and translational research is encouraging adoption of specialized and single-use cell culture solutions.
Germany Cell Culture Media & Single-Use Reagents Market Insight
The Germany cell culture media & single-use reagents market is expected to expand at a considerable CAGR during the forecast period, fueled by the country’s strong pharmaceutical manufacturing base and emphasis on innovation and quality. Germany is a major hub for biologics production and bioprocessing technologies, driving demand for GMP-grade media and reagents. The country’s well-developed research infrastructure and strong collaboration between industry and academia further support market growth. Additionally, increasing adoption of single-use systems to improve efficiency and regulatory compliance is contributing to sustained demand.
Asia-Pacific Cell Culture Media & Single-Use Reagents Market Insight
The Asia-Pacific cell culture media & single-use reagents market is poised to grow at the fastest CAGR during the forecast period, driven by expanding pharmaceutical manufacturing capacity, rising investments in biotechnology, and growing CRO and CDMO activities. Countries such as China, Japan, South Korea, and India are witnessing rapid growth in biologics production and clinical research. Government initiatives supporting life sciences innovation and healthcare infrastructure development are further accelerating adoption. The region’s emergence as a global manufacturing and outsourcing hub is significantly boosting demand for cost-effective yet high-quality media and reagents.
Japan Cell Culture Media & Single-Use Reagents Market Insight
The Japan cell culture media & single-use reagents market is gaining momentum due to the country’s advanced healthcare system, strong focus on innovation, and growing investment in regenerative medicine. Japan is a global leader in stem cell research and cell-based therapies, driving demand for specialized media and single-use reagents. The increasing number of research collaborations and clinical trials further supports market growth. Additionally, the emphasis on quality, precision, and regulatory compliance aligns well with the adoption of advanced cell culture solutions across research and manufacturing settings.
India Cell Culture Media & Single-Use Reagents Market Insight
The India cell culture media & single-use reagents market accounted for a significant revenue share within Asia-Pacific in 2025, driven by rapid expansion of the pharmaceutical and biotechnology sectors. India’s growing role as a global hub for biologics manufacturing, biosimilars, and contract research is fueling demand for cell culture media and reagents. Increasing investments in life sciences research, rising clinical trial activity, and supportive government initiatives are key growth drivers. Additionally, the presence of cost-effective manufacturing capabilities and expanding CRO/CDMO services is accelerating adoption across research and commercial applications.
Cell Culture Media & Single-Use Reagents Market Share
The Cell Culture Media & Single-Use Reagents industry is primarily led by well-established companies, including:
- Thermo Fisher Scientific Inc. (U.S.)
- Lonza Ltd (Switzerland)
- Corning Incorporated (U.S.)
- Sartorius AG (Germany)
- Merck KGaA (Germany)
- BD (U.S.)
- HiMedia Laboratories Pvt. Ltd. (India)
- FUJIFILM Irvine Scientific, Inc. (U.S.)
- ATCC (U.S.)
- Bio Techne Corporation (U.S.)
- CLS Cell Lines Service GmbH (Germany)
- InvivoGen Corp. (France)
- Kohjin Bio Co. Ltd. (Japan)
- LGC Science Group Holdings Ltd (U.K.)
- Miltenyi Biotec B.V. & Co. KG (Germany)
- PromoCell GmbH (Germany)
- Takara Bio Inc. (Japan)
- ZenBio Inc. (U.S.)
- CellGenix GmbH (Germany)
- PeproTech, Inc. (U.S.)
What are the Recent Developments in Global Cell Culture Media & Single-Use Reagents Market?
- In November 2024, Agilent Technologies restructured its Life Sciences and Diagnostics Markets Group to intensify focus on cell‑based solutions including single‑cell analysis, genomics, and cell culture technologies. This realignment aims to enhance Agilent’s innovation capabilities in cell therapy and drug discovery platforms, supporting researchers and biopharma companies with advanced analytical and culture tools crucial for next‑generation therapeutic development
- In April 2024, Thermo Fisher Scientific introduced the Gibco™ CTS™ OpTmizer™ One Serum‑Free Medium (SFM), a ready‑to‑use animal origin‑free formulation specifically developed for clinical and commercial T cell expansion. This innovation delivers increased scalability and performance for cell therapy manufacturing, enabling improved viability and early memory T‑cell phenotype while reducing variability from animal components, thereby helping manufacturers streamline workflows and accelerate time to patient delivery
- In March 2024, Merck KGaA announced a major investment of over €300 million to establish a new Life Science Research Center dedicated to advancing cell biology technologies that underpin drug discovery, regenerative medicine, and cell therapy production. The center will serve as a hub for developing next‑generation media and reagent solutions while fostering collaborations with academic and industry partners to accelerate scientific progress
- In July 2023, PromoCell introduced the PromoExQ MSC Growth Medium XF, a serum‑ and xeno‑free medium tailored for GMP‑compliant expansion of mesenchymal stem cells (MSCs). This product supports long‑term MSC culture with consistent growth rates and quality, addressing the increasing need for standardized media in stem cell therapy research and manufacturing. Its GMP certification ensures reliability for clinical applications and enhanced reproducibility for therapeutic development
- In May 2023, Lonza, a leading global biotech company, launched the TheraPEAK® T‑VIVO® Cell Culture Medium, a novel chemically defined medium designed to optimize CAR‑T cell manufacturing by eliminating animal‑derived components, improving process consistency, and simplifying regulatory compliance for accelerated therapeutic development. This launch supports the growing demand for specialized media tailored to cell therapy workflows and enhances quality control across clinical and commercial production stages
SKU-
世界初のマーケットインテリジェンスクラウドに関するレポートにオンラインでアクセスする
- インタラクティブなデータ分析ダッシュボード
- 成長の可能性が高い機会のための企業分析ダッシュボード
- カスタマイズとクエリのためのリサーチアナリストアクセス
- インタラクティブなダッシュボードによる競合分析
- 最新ニュース、更新情報、トレンド分析
- 包括的な競合追跡のためのベンチマーク分析のパワーを活用
調査方法
データ収集と基準年分析は、大規模なサンプル サイズのデータ収集モジュールを使用して行われます。この段階では、さまざまなソースと戦略を通じて市場情報または関連データを取得します。過去に取得したすべてのデータを事前に調査および計画することも含まれます。また、さまざまな情報ソース間で見られる情報の不一致の調査も含まれます。市場データは、市場統計モデルと一貫性モデルを使用して分析および推定されます。また、市場シェア分析と主要トレンド分析は、市場レポートの主要な成功要因です。詳細については、アナリストへの電話をリクエストするか、お問い合わせをドロップダウンしてください。
DBMR 調査チームが使用する主要な調査方法は、データ マイニング、データ変数が市場に与える影響の分析、および一次 (業界の専門家) 検証を含むデータ三角測量です。データ モデルには、ベンダー ポジショニング グリッド、市場タイムライン分析、市場概要とガイド、企業ポジショニング グリッド、特許分析、価格分析、企業市場シェア分析、測定基準、グローバルと地域、ベンダー シェア分析が含まれます。調査方法について詳しくは、お問い合わせフォームから当社の業界専門家にご相談ください。
カスタマイズ可能
Data Bridge Market Research は、高度な形成的調査のリーダーです。当社は、既存および新規のお客様に、お客様の目標に合致し、それに適したデータと分析を提供することに誇りを持っています。レポートは、対象ブランドの価格動向分析、追加国の市場理解 (国のリストをお問い合わせください)、臨床試験結果データ、文献レビュー、リファービッシュ市場および製品ベース分析を含めるようにカスタマイズできます。対象競合他社の市場分析は、技術ベースの分析から市場ポートフォリオ戦略まで分析できます。必要な競合他社のデータを、必要な形式とデータ スタイルでいくつでも追加できます。当社のアナリスト チームは、粗い生の Excel ファイル ピボット テーブル (ファクト ブック) でデータを提供したり、レポートで利用可能なデータ セットからプレゼンテーションを作成するお手伝いをしたりすることもできます。