中東およびアフリカの電子臨床ソリューション市場、製品別(電子データキャプチャおよび臨床試験データ管理システム、臨床試験管理システム、臨床分析プラットフォーム、ケアコーディネーション医療記録(CCMR)、ランダム化および試験供給管理、臨床データ統合プラットフォーム、電子臨床結果評価ソリューション、安全性ソリューション、電子試験マスターファイルシステム、規制情報管理ソリューションなど)、配信モード(Webホスト(オンデマンド)ソリューション、ライセンスエンタープライズ(オンプレミス)ソリューション、クラウドベース(SAAS)ソリューション)、臨床試験フェーズ(フェーズI、フェーズII、フェーズIII、フェーズIV)、組織規模(小規模および中規模および大規模)、ユーザーデバイス(デスクトップ、タブレット、ハンドヘルドPDAデバイス、スマートフォンなど)、エンドユーザー(製薬会社およびバイオ医薬品会社、契約研究機関、コンサルティングサービス会社、医療機器メーカー、病院、学術研究機関)、業界動向および2030年までの予測。
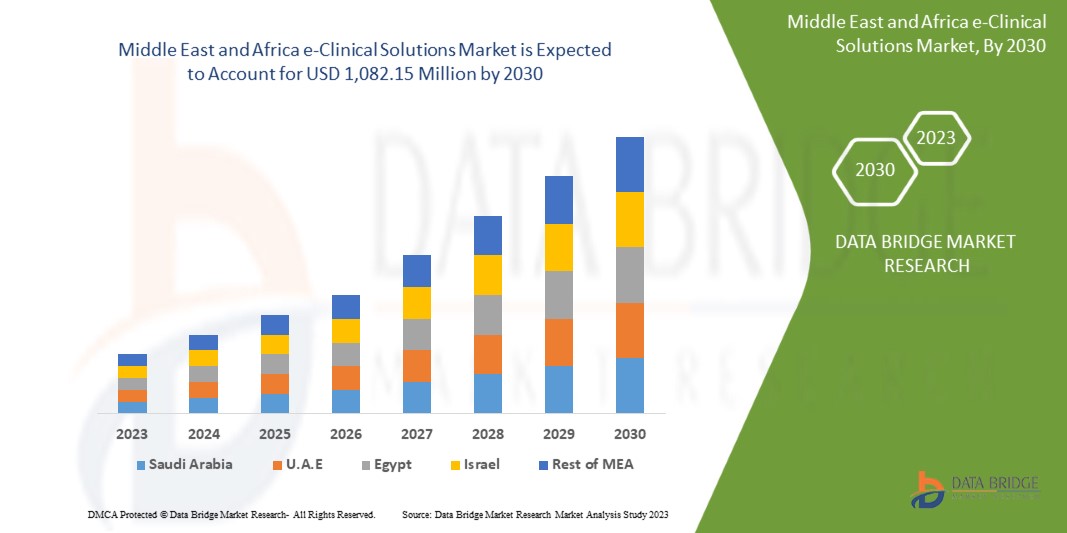
中東およびアフリカのe-Clinicalソリューション市場の分析と規模
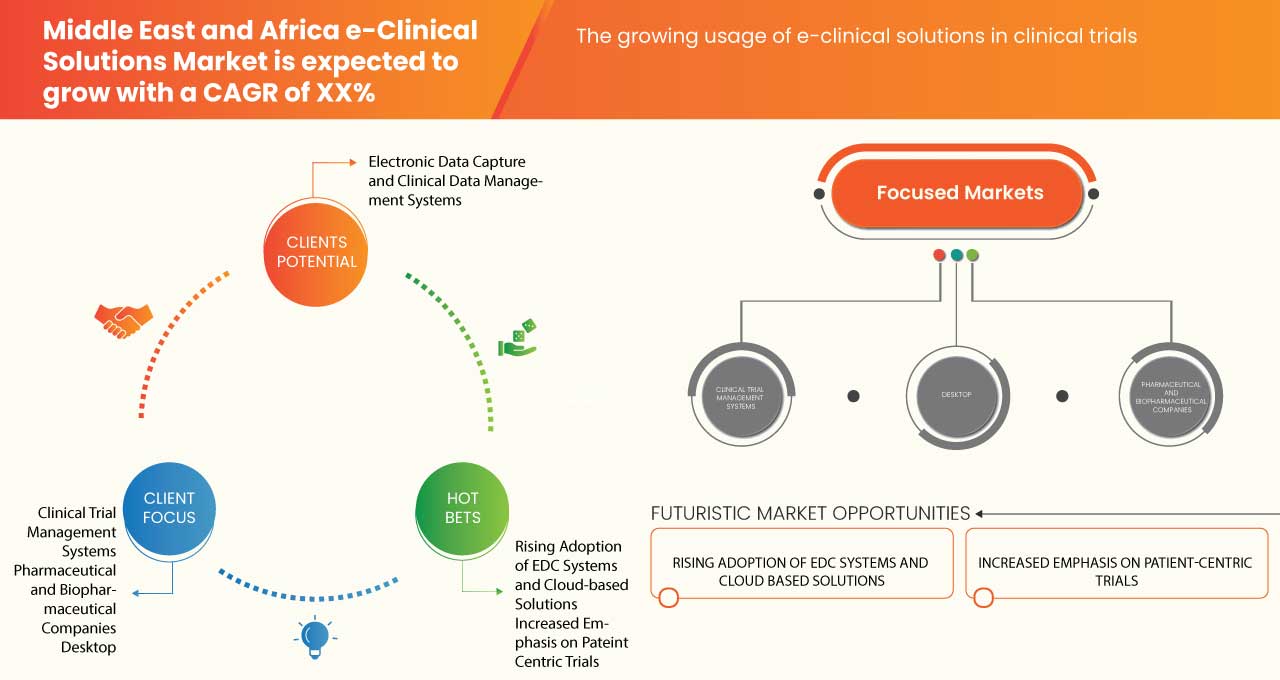
中東およびアフリカの電子臨床ソリューション市場は、Oracle、IQVIA Inc.、Dassault Systemes、Clario など、多くのグローバル プレーヤーで構成されているため、細分化されています。これらの企業の存在により、地域全体でシステム サービスとソフトウェアの価格が競争力のあるものになっています。地域レベルおよび国際レベルでこれらのプレーヤーが存在するため、サプライヤーとメーカーは、あらゆる予算でさまざまな仕様と特性を持つ製品を提供しています。電子データ キャプチャ (EDC) システムとクラウドベースの採用の増加が、市場の成長を牽引しています。さらに、患者中心の臨床試験への注目の高まりも、市場の成長を牽引すると予想されます。
Data Bridge Market Research の分析によると、中東およびアフリカの e 臨床ソリューション市場は、予測期間中に 11.9% の CAGR で成長し、2030 年までに 10 億 8,215 万米ドルに達すると予想されています。この市場レポートでは、価格分析と技術の進歩についても詳細に取り上げています。
|
レポートメトリック |
詳細 |
|
予測期間 |
2023年から2030年 |
|
基準年 |
2022 |
|
歴史的な年 |
2021(2015~2020年にカスタマイズ可能) |
|
定量単位 |
売上高は百万米ドル、販売数量は個数、価格は米ドル |
|
対象セグメント |
製品別 (電子データキャプチャおよび臨床試験データ管理システム、臨床試験管理システム、臨床分析プラットフォーム、ケアコーディネーション医療記録 (CCMR)、ランダム化および試験供給管理、臨床データ統合プラットフォーム、電子臨床結果評価ソリューション、安全性ソリューション、電子試験マスターファイルシステム、規制情報管理ソリューションなど)、提供モード別 (Web ホスト型 (オンデマンド) ソリューション、ライセンスを受けたエンタープライズ (オンプレミス) ソリューション、クラウドベース (SAAS) ソリューション)、臨床試験フェーズ別 (フェーズ I、フェーズ II、フェーズ III、フェーズ IV)、組織規模別 (小規模、中規模、大規模)、ユーザーデバイス別 (デスクトップ、タブレット、ハンドヘルド PDA デバイス、スマートフォンなど)、エンドユーザー別 (製薬会社、バイオ医薬品会社、開発業務委託機関、コンサルティングサービス会社、医療機器メーカー、病院、学術研究機関) |
|
対象国 |
南アフリカ、サウジアラビア、UAE、エジプト、イスラエル、その他の中東およびアフリカ諸国 |
|
対象となる市場プレーヤー |
Oracle、Signant Health、MaxisIT、Paraxel International Corporation、Dassault Systemes、Clario、Mednet、OpenClinica、LLC、4G Clinical、Veeva Systems、Saama Technologies、LLC、Anju、Castor、Medrio、Inc.、ArisGlobal、Merative、Advarra、eClinical Solutions、LLC、Y-Prime LLC、RealTime Software Solutions LLC、Quretec、Research Manager、Datatrack Int.、および IQVIA Inc.
|
市場の定義
e-clinical ソリューションの目標は、臨床研究の分野に革命を起こすことです。臨床管理組織は、患者をより迅速に治療するために、臨床研究データの有効性、効率性、およびアクセス性を改善しようとしています。eClinical ソリューションの作成の背後にあるアイデアは、臨床研究データの問題を特定して修正し、臨床試験データを有用かつ簡単に取得できるようにするための独創的なソリューションを提供し、臨床研究分野の標準化、レポート、および運用を促進することでした。e-clinical ソリューションとソフトウェアの採用の増加と注目の高まりにより、市場の成長が促進されると予想されます。
電子データキャプチャ(EDC)、電子患者報告アウトカム(ePRO)、臨床試験管理システム(CTMS)、電子試験マスターファイル(eTMF)システムなどのe臨床ソリューションは、臨床研究で臨床データを管理するために使用されるソフトウェアプログラムです。ただし、臨床試験研究の承認と製品の承認に関する厳格な規制と基準により、市場の成長が抑制されると予想されます。
中東およびアフリカのe-Clinicalソリューション市場の動向
このセクションでは、市場の推進要因、機会、制約、課題について理解します。これらについては、以下で詳しく説明します。
ドライバー
研究開発活動とそれに伴う支出の増加
Research & development is crucial in all industries, including life science and healthcare. The life science industry carries out extensive research and development to generate revenue and often brings results that embrace saving or enhancing a patient's life. Drug development requires going through clinical trials to check clinical data analysis and review its study and new data insights.
The e-clinical solutions are used for comprehensive data review, automating the data transformation process, and analytics to accelerate timelines. Pharmaceutical and biopharmaceutical companies conduct many clinical trials to complete successful drug or biologic development. Pharmaceutical and biopharmaceutical companies have increased their focus on research and development, raising the demand for e-clinical solutions.
Improved healthcare infrastructure and establishment of advanced laboratories
Infrastructure is a key pillar supporting the fundamental aim of promoting improved standards of care and well-being for all patients, together with a good healthcare system experience. In parallel, the healthcare system and staff must support effective health promotion, prevention, and self-care of the whole population.
Infrastructure must integrate the hospital, as the acute and in-patient care center, into the broader healthcare system. It should facilitate the seven domains of quality patient experience, effectiveness, efficiency, timeliness, safety, equity, and sustainability. Infrastructure includes the built environment and supporting elements such as equipment, access, information technology (IT), systems and processes, sustainability initiatives, and staff. This improved healthcare infrastructure and staff more opportunities for e-clinical solutions to grow and give better results.
OPPORTUNITY
Increasing investments by various governments in clinical trials
Clinical trial funding comes from various sources, including the government, commercial investors, nonprofit organizations, academic institutions, and other research organizations. Historically, the National Institutes of Health (NIH) has made the biggest government investments in fundamental drug development research. The defense advanced research agency (DARPA) has also contributed to the discovery stage by accepting a few relatively high-risk biologic initiatives. State governments are also increasingly taking the lead in this area, partly due to the public's frustration with the lengthy discovery process. Thus, government initiatives and investments toward discovering new drugs with trial studies are expected to create an opportunity for market growth.
RESTRAINT / CHALLENGE
Data Safety and Privacy issues
Information systems are heavily concerned with privacy and security. Access to individual health information is made possible by digitalizing healthcare services from any electronic device with an internet connection anywhere globally. Users typically are unaware of how their data is handled. Healthcare data on the cloud has caught the attention of hackers, who target systems to launch attacks and steal sensitive data in exchange for financial benefit.
Data breaches have been common since 2019, over 50 million electronic medical records have been hacked in 2021, and the number of security breaches was predicted to increase every year. The creation of inter-jurisdictional data-sharing agreements and the storage and manipulation of data holdings are significantly hampered by concerns about personal privacy and information confidentiality and the recent enactment of privacy and confidentiality legislation across the provinces and territories (exceptionally patient records). Thus, it is a restraint for the Middle East and Africa e-clinical solutions market and is expected to hamper market growth.
Post-COVID-19 Impact on the Middle East and Africa e-Clinical Solutions Market
COVID-19 positively impacted the Middle East and Africa e-clinical solutions market. The lockdown restriction led to the emergence of various opportunities such as drug development, EMR and EHR application installation, and many others.
However, increasing government support and advanced and innovative techniques are expected to provide lucrative opportunities for market growth. Moreover, increasing partnerships, acquisitions, and collaboration among market players are expected to further fuel market growth. Also, the growth has been high since the market opened after COVID-19, and it is expected that there will be considerable growth in the sector. The market players are conducting multiple activities to improve the trial study techniques. With this, the companies will bring advancement and innovation to the market.
Recent Developments
- In April 2023, the Medidata subsidiary of Dassault Systèmes announced Lambda Therapeutics is deploying Medidata's cloud-based clinical products, Rave EDC, Rave RTSM, and Rave Imaging, according to a statement from Dassault Systèmes subsidiary Medidata. Automating and optimizing data management operations and securely delivering higher-quality data for quicker insights will further improve clinical trial productivity. This has aided the business in promoting its offerings across the globe
- In March 2023, Clario has launched a cloud-based Image Viewer tool that helps the Sponsors and CROs to see the images of their clinical trials. Previously, several organizations had to participate in the image transfer procedure to see photos for a clinical trial. This complicated the already risky process and increased the possibility of delays and mistakes. This has aided the business in growing its service offering
Middle East and Africa e-Clinical Solutions Market Scope
The Middle East and Africa e-clinical solutions market is segmented into six notable segments based on product, delivery mode, clinical trial phase, organization size, user device, and end user. The growth amongst these segments will help you analyze meager growth segments in the industries and provide the users with a valuable market overview and insights to help them make strategic decisions for identifying core market applications.
Product
- Electronic Data Capture and Clinical Data Management Systems
- Clinical Trial Management Systems
- Clinical Analytics Platforms
- Care Coordination Medical Record (CCMR)
- Randomization and Trial Supply Management
- Clinical Data Integration Platforms
- Electronic Clinical Outcome Assessment Solutions
- Safety Solutions
- Electronic Trial Master File Systems
- Regulatory Information Management Solutions
- Others
Based on product, the market is segmented into electronic data capture and clinical data management, clinical trial management systems, clinical analytics platforms, care coordination medical record (CCMR), randomization and trial supply management, clinical data integration platforms, electronic clinical outcome assessment solutions, safety solutions, electronic trial master file systems, regulatory information management solutions and others.
Delivery Mode
- Web-Hosted (On-Demand) Solutions
- Licensed Enterprise (On-Premises) Solutions
- Cloud-based (SAAS) Solutions
Based on delivery mode, the market is segmented into web-hosted (on-demand) solutions, licensed enterprise (on-premises) solutions, and cloud-based (SAAS) solutions.
Clinical Trial Phase
- Phase I
- Phase II
- Phase III
- Phase IV
Based on clinical trial phase, the market is segmented into Phase I, Phase II, Phase III, and Phase IV.
Organization Size
- Small & Medium
- Large
Based on organization size, the market is segmented into small & medium, and large.
User Device
- Desktop
- Tablet
- Handheld PDA Device
- Smart Phone
- Others
Based on user device, the market is segmented into desktop, tablet, handheld PDA device, smart phone, and others.
End User
- Pharmaceutical and Biopharmaceutical companies
- Contract Research Organizations
- Consulting Service Companies
- Medical Device Manufacturers
- Hospitals
- Academic Research Institutes
Based on end user, the market is segmented into pharmaceutical and biopharmaceutical companies, contract research organizations, consulting service companies, medical device manufacturers, hospitals, and academic research institutes.
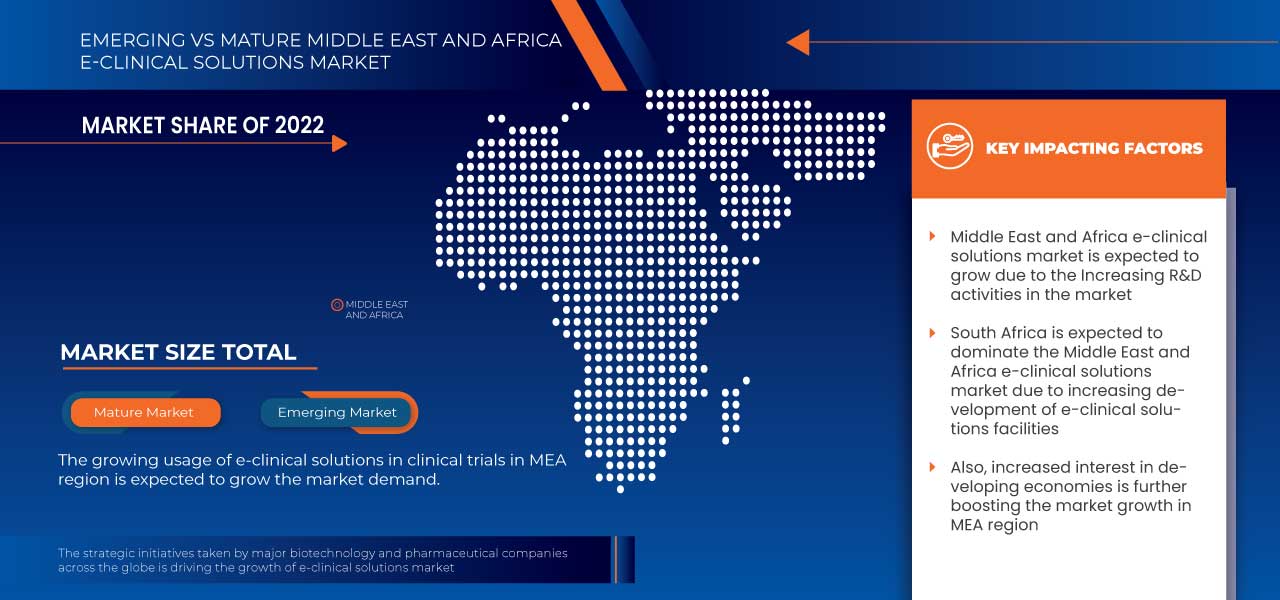
Middle East and Africa e-Clinical Solutions Market Regional Analysis/Insights
The Middle East and Africa e-clinical solutions market is analyzed, and market size information is p based on product, delivery mode, clinical trial phase, organization size, user device, and end user.
The countries covered in this market report are South Africa, Saudi Arabia, U.A.E., Egypt, Israel, and the Rest of the Middle East and Africa.
南アフリカは、電子データキャプチャシステムとクラウドベースのソリューションの導入の増加により、中東およびアフリカ地域を支配すると予想されています。
レポートの国別セクションでは、市場の現在および将来の傾向に影響を与える国内市場における個別の市場影響要因と規制の変更も提供しています。新規販売、交換販売、国の人口統計、規制行為、輸出入関税などのデータポイントは、個々の国の市場シナリオを予測するために使用される主要な指標の一部です。また、国別データの予測分析を提供する際には、アジア太平洋ブランドの存在と入手可能性、地元および国内ブランドとの競争が激しいか少ないために直面する課題、販売チャネルの影響も考慮されています。
競争環境と中東およびアフリカのe-Clinicalソリューション市場シェア分析
中東およびアフリカの電子臨床ソリューション市場の競争状況は、競合他社の詳細を提供します。詳細には、会社概要、財務、収益、市場の可能性、新しい市場への取り組み、世界的なプレゼンス、生産拠点と施設、生産能力、会社の強みと弱み、製品の発売、製品の幅と広さ、アプリケーションの優位性が含まれます。提供されている上記のデータ ポイントは、市場に関連する会社の焦点にのみ関連しています。
中東およびアフリカの電子臨床ソリューション市場で活動している主要な市場プレーヤーには、Oracle、Signant Health、MaxisIT、Paraxel International Corporation、Dassault Systemes、Clario、Mednet、OpenClinica、LLC、4G Clinical、Veeva Systems、Saama Technologies、LLC、Anju、Castor、Medrio、Inc.、ArisGlobal、Merative、Advarra、eClinical Solutions、LLC、Y-Prime LLC、RealTime Software Solutions などがあります。
SKU-
世界初のマーケットインテリジェンスクラウドに関するレポートにオンラインでアクセスする
- インタラクティブなデータ分析ダッシュボード
- 成長の可能性が高い機会のための企業分析ダッシュボード
- カスタマイズとクエリのためのリサーチアナリストアクセス
- インタラクティブなダッシュボードによる競合分析
- 最新ニュース、更新情報、トレンド分析
- 包括的な競合追跡のためのベンチマーク分析のパワーを活用
目次
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW
1.4 CURRENCY AND PRICING
1.5 LIMITATIONS
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 DBMR TRIPOD DATA VALIDATION MODEL
2.5 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.6 MULTIVARIATE MODELLING
2.7 MARKET END USER COVERAGE GRID
2.8 PRODUCT LIFELINE CURVE
2.9 DBMR MARKET POSITION GRID
2.1 VENDOR SHARE ANALYSIS
2.11 SECONDARY SOURCES
2.12 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PORTER'S FIVE FORCES MODEL
4.2 ECOSYSTEMS ANALYSIS OF E CLINICAL SOLUTIONS
4.3 USE CASES
5 VALUE CHAIN ANALYSIS
6 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: REGULATIONS
7 MARKET OVERVIEW
7.1 DRIVERS
7.1.1 THE GROWING USAGE OF E-CLINICAL SOLUTIONS IN CLINICAL TRIALS
7.1.2 INCREASING R&D ACTIVITIES AND CORRESPONDING EXPENDITURE
7.1.3 STRATEGIC INITIATIVES TAKEN BY MAJOR BIOTECHNOLOGY AND PHARMACEUTICAL COMPANIES
7.1.4 IMPROVED HEALTHCARE INFRASTRUCTURE AND ESTABLISHMENT OF ADVANCED LABORATORIES
7.2 RESTRAINTS
7.2.1 DATA SAFETY AND PRIVACY ISSUES
7.2.2 LACK OF SKILLED PROFESSIONALS
7.2.3 LIMITED ADOPTION OF E-CLINICAL SOLUTIONS IN EMERGING COUNTRIES DUE TO BUDGETARY CONSTRAINTS AND POOR MANAGEMENT POLICIES
7.3 OPPORTUNITIES
7.3.1 RISING ADOPTION OF ELECTRONIC DATA CAPTURE (EDC) SYSTEMS AND CLOUD-BASED SOLUTIONS
7.3.2 INCREASING INVESTMENTS BY VARIOUS GOVERNMENTS IN CLINICAL TRIALS
7.3.3 GROWING FOCUS ON PATIENT-CENTRIC CLINICAL TRIALS
7.4 CHALLENGES
7.4.1 REGULATORY CHALLENGES ASSOCIATED WITH E-CLINICAL SOLUTIONS
7.4.2 HIGH IMPLEMENTATION COSTS
8 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET, BY PRODUCT
8.1 OVERVIEW
8.2 ELECTRONIC DATA CAPTURE AND CLINICAL DATA MANAGEMENT SYSTEMS
8.3 RANDOMIZATION AND TRIAL SUPPLY MANAGEMENT
8.4 CLINICAL TRIAL MANAGEMENT SYSTEMS
8.5 ELECTRONIC TRIAL MASTER FILE SYSTEMS
8.6 ELECTRONIC CLINICAL OUTCOME ASSESSMENT SOLUTIONS
8.7 SAFETY SOLUTIONS
8.8 REGULATORY INFORMATION MANAGEMENT SOLUTIONS
8.9 CLINICAL DATA INTEGRATION PLATFORMS
8.1 CLINICAL ANALYTICS PLATFORMS
8.11 CARE COORDINATION MEDICAL RECORD (CCMR)
8.12 OTHERS
9 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET, BY DELIVERY MODE
9.1 OVERVIEW
9.2 WEB-HOSTED (ON-DEMAND) SOLUTIONS
9.3 CLOUD-BASED (SAAS) SOLUTIONS
9.4 LICENSED ENTERPRISE (ON-PREMISES) SOLUTIONS
10 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET, BY CLINICAL TRIAL PHASE
10.1 OVERVIEW
10.2 PHASE III
10.3 PHASE I
10.4 PHASE II
10.5 PHASE IV
11 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET, BY ORGANIZATION SIZE
11.1 OVERVIEW
11.2 MEDIUM AND SMALL
11.3 LARGE
12 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET, BY USER DEVICE
12.1 OVERVIEW
12.2 DESKTOP
12.3 TABLET
12.4 SMART PHONE
12.5 HANDHELD PDA DEVICE
12.6 OTHERS
13 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET, BY END USER
13.1 OVERVIEW
13.2 CONTRACT RESEARCH ORGANIZATIONS
13.3 PHARMACEUTICAL AND BIOPHARMACEUTICAL COMPANIES
13.4 MEDICAL DEVICE MANUFACTURERS
13.5 HOSPITAL
13.6 CONSULTING SERVICE COMPANIES
13.7 ACADEMIC RESEARCH INSTITUTES
14 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET, BY REGION
14.1 MIDDLE EAST AND AFRICA
14.1.1 SOUTH AFRICA
14.1.2 SAUDI ARABIA
14.1.3 U.A.E.
14.1.4 EGYPT
14.1.5 ISRAEL
14.1.6 REST OF MIDDLE EAST AND AFRICA
15 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET, COMPANY LANDSCAPE
15.1 COMPANY SHARE ANALYSIS: MIDDLE EAST & AFRICA
16 SWOT ANALYSIS
17 COMPANY PROFILE
17.1 ORACLE
17.1.1 COMPANY SNAPSHOT
17.1.2 REVENUE ANALYSIS
17.1.3 COMPANY SHARE ANALYSIS
17.1.4 PRODUCT PORTFOLIO
17.1.5 RECENT DEVELOPMENT
17.2 DASSAULT SYSTEMES
17.2.1 COMPANY SNAPSHOT
17.2.2 REVENUE ANALYSIS
17.2.3 COMPANY SHARE ANALYSIS
17.2.4 PRODUCT PORTFOLIO
17.2.5 RECENT DEVELOPMENTS
17.3 CLARIO
17.3.1 COMPANY SNAPSHOT
17.3.2 COMPANY SHARE ANALYSIS
17.3.3 PRODUCT PORTFOLIO
17.3.4 RECENT DEVELOPMENT
17.4 VEEVA SYSTEMS
17.4.1 COMPANY SNAPSHOT
17.4.2 REVENUE ANALYSIS
17.4.3 COMPANY SHARE ANALYSIS
17.4.4 PRODUCT PORTFOLIO
17.4.5 RECENT DEVELOPMENTS
17.5 ARISMIDDLE EAST & AFRICA
17.5.1 COMPANY SNAPSHOT
17.5.2 COMPANY SHARE ANALYSIS
17.5.3 PRODUCT PORTFOLIO
17.5.4 RECENT DEVELOPMENTS
17.6 4G CLINICAL
17.6.1 COMPANY SNAPSHOT
17.6.2 PRODUCT PORTFOLIO
17.6.3 RECENT DEVELOPMENT
17.7 ADVARRA.
17.7.1 COMPANY SNAPSHOT
17.7.2 PRODUCT PORTFOLIO
17.7.3 RECENT DEVELOPMENT
17.8 ANJU
17.8.1 COMPANY SNAPSHOT
17.8.2 PRODUCT PORTFOLIO
17.8.3 RECENT DEVELOPMENTS
17.9 CASTOR.
17.9.1 COMPANY SNAPSHOT
17.9.2 PRODUCT PORTFOLIO
17.9.3 RECENT DEVELOPMENTS
17.1 DATATRAK INT.
17.10.1 COMPANY SNAPSHOT
17.10.2 PRODUCT PORTFOLIO
17.10.3 RECENT DEVELOPMENT
17.11 ECLINICAL SOLUTIONS LLC.
17.11.1 COMPANY SNAPSHOT
17.11.2 PRODUCT PORTFOLIO
17.11.3 RECENT DEVELOPMENT
17.12 IQVIA INC
17.12.1 COMPANY SNAPSHOT
17.12.2 REVENUE ANALYSIS
17.12.3 PRODUCT PORTFOLIO
17.12.4 RECENT DEVELOPMENTS
17.13 MAXISIT
17.13.1 COMPANY SNAPSHOT
17.13.2 PRODUCT PORTFOLIO
17.13.3 RECENT DEVELOPMENT
17.14 MEDNET
17.14.1 COMPANY SNAPSHOT
17.14.2 PRODUCT PORTFOLIO
17.14.3 RECENT DEVELOPMENT
17.15 MEDRIO, INC.
17.15.1 COMPANY SNAPSHOT
17.15.2 PRODUCT PORTFOLIO
17.15.3 RECENT DEVELOPMENTS
17.16 MERATIVE
17.16.1 COMPANY SNAPSHOT
17.16.2 PRODUCT PORTFOLIO
17.16.3 RECENT DEVELOPMENT
17.17 OPENCLINICA, LLC
17.17.1 COMPANY SNAPSHOT
17.17.2 PRODUCT PORTFOLIO
17.17.3 RECENT DEVELOPMENT
17.18 PAREXEL INTERNATIONAL CORPORATION
17.18.1 COMPANY SNAPSHOT
17.18.2 PRODUCT PORTFOLIO
17.18.3 RECENT DEVELOPMENT
17.19 QURETEC
17.19.1 COMPANY SNAPSHOT
17.19.2 PRODUCT PORTFOLIO
17.19.3 RECENT DEVELOPMENT
17.2 REALTIME SOFTWARE SOLUTIONS, LLC
17.20.1 COMPANY SNAPSHOT
17.20.2 PRODUCT PORTFOLIO
17.20.3 RECENT DEVELOPMENT
17.21 RESEARCH MANAGER
17.21.1 COMPANY SNAPSHOT
17.21.2 PRODUCT PORTFOLIO
17.21.3 RECENT DEVELOPMENT
17.22 SAAMA TECHNOLOGIES, LLC
17.22.1 COMPANY SNAPSHOT
17.22.2 PRODUCT PORTFOLIO
17.22.3 RECENT DEVELOPMENT
17.23 SIGNANT HEALTH
17.23.1 COMPANY SNAPSHOT
17.23.2 PRODUCT PORTFOLIO
17.23.3 RECENT DEVELOPMENT
17.24 Y-PRIME, LLC.
17.24.1 COMPANY SNAPSHOT
17.24.2 PRODUCT PORTFOLIO
17.24.3 RECENT DEVELOPMENT
18 QUESTIONNAIRE
19 RELATED REPORTS
表のリスト
TABLE 1 THE COSTS OF IMPLEMENTATION ARE AS FOLLOWS, ACCORDING TO SIMPLETRIALS:
TABLE 2 ACCORDING TO ECLINICAL WORKS – ECLINICAL OFFERS TWO ENTERPRISE PRICING PACKAGES:
TABLE 3 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 4 MIDDLE EAST & AFRICA ELECTRONIC DATA CAPTURE AND CLINICAL DATA MANAGEMENT SYSTEMS IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 5 MIDDLE EAST & AFRICA RANDOMIZATION AND TRIAL SUPPLY MANAGEMENT IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 6 MIDDLE EAST & AFRICA CLINICAL TRIAL MANAGEMENT SYSTEMS IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 7 MIDDLE EAST & AFRICA ELECTRONIC TRIAL MASTER FILE SYSTEMS IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 8 MIDDLE EAST & AFRICA ELECTRONIC CLINICAL OUTCOME ASSESSMENT SOLUTIONS IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 9 MIDDLE EAST & AFRICA SAFETY SOLUTIONS IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 10 MIDDLE EAST & AFRICA REGULATORY INFORMATION MANAGEMENT SOLUTIONS IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 11 MIDDLE EAST & AFRICA CLINICAL DATA INTEGRATION PLATFORMS IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 12 MIDDLE EAST & AFRICA CLINICAL ANALYTICS PLATFORMS IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 13 MIDDLE EAST & AFRICA CARE COORDINATION MEDICAL RECORD (CCMR) IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 14 MIDDLE EAST & AFRICA OTHERS IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 15 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET, BY DELIVERY MODE, 2021-2030 (USD MILLION)
TABLE 16 MIDDLE EAST & AFRICA WEB-HOSTED (ON-DEMAND) SOLUTIONS IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 17 MIDDLE EAST & AFRICA CLOUD-BASED (SAAS) SOLUTIONS IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 18 MIDDLE EAST & AFRICA LICENSED ENTERPRISE (ON-PREMISES) SOLUTIONS IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 19 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET, BY CLINICAL TRIAL PHASE, 2021-2030 (USD MILLION)
TABLE 20 MIDDLE EAST & AFRICA PHASE III IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 21 MIDDLE EAST & AFRICA PHASE I IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 22 MIDDLE EAST & AFRICA PHASE II IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 23 MIDDLE EAST & AFRICA PHASE IV IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 24 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET, BY ORGANIZATION SIZE, 2021-2030 (USD MILLION)
TABLE 25 MIDDLE EAST & AFRICA MEDIUM AND SMALL IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 26 MIDDLE EAST & AFRICA LARGE IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 27 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET, BY USER DEVICE, 2021-2030 (USD MILLION)
TABLE 28 MIDDLE EAST & AFRICA DESKTOP IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 29 MIDDLE EAST & AFRICA TABLET IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 30 MIDDLE EAST & AFRICA SMART PHONE IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 31 MIDDLE EAST & AFRICA HANDHELD PDA DEVICE IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 32 MIDDLE EAST & AFRICA OTHERS IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 33 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 34 MIDDLE EAST & AFRICA CONTRACT RESEARCH ORGANIZATIONS IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 35 MIDDLE EAST & AFRICA PHARMACEUTICAL AND BIOPHARMACEUTICAL COMPANIES IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 36 MIDDLE EAST & AFRICA MEDICAL DEVICE MANUFACTURERS IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 37 MIDDLE EAST & AFRICA HOSPITAL IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 38 MIDDLE EAST & AFRICA CONSULTING SERVICE COMPANIES IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 39 MIDDLE EAST & AFRICA ACADEMIC RESEARCH INSTITUTES IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 40 MIDDLE EAST AND AFRICA E-CLINICAL SOLUTIONS MARKET, BY COUNTRY, 2021-2030 (USD MILLION)
TABLE 41 MIDDLE EAST AND AFRICA E-CLINICAL SOLUTIONS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 42 MIDDLE EAST AND AFRICA E-CLINICAL SOLUTIONS MARKET, BY DELIVERY MODE, 2021-2030 (USD MILLION)
TABLE 43 MIDDLE EAST AND AFRICA E-CLINICAL SOLUTIONS MARKET, BY CLINICAL TRIAL PHASE, 2021-2030 (USD MILLION)
TABLE 44 MIDDLE EAST AND AFRICA E-CLINICAL SOLUTIONS MARKET, BY ORGANIZATION SIZE, 2021-2030 (USD MILLION)
TABLE 45 MIDDLE EAST AND AFRICA E-CLINICAL SOLUTIONS MARKET, BY USER DEVICE, 2021-2030 (USD MILLION)
TABLE 46 MIDDLE EAST AND AFRICA E-CLINICAL SOLUTIONS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 47 SOUTH AFRICA E-CLINICAL SOLUTIONS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 48 SOUTH AFRICA E-CLINICAL SOLUTIONS MARKET, BY DELIVERY MODE, 2021-2030 (USD MILLION)
TABLE 49 SOUTH AFRICA E-CLINICAL SOLUTIONS MARKET, BY CLINICAL TRIAL PHASE, 2021-2030 (USD MILLION)
TABLE 50 SOUTH AFRICA E-CLINICAL SOLUTIONS MARKET, BY ORGANIZATION SIZE, 2021-2030 (USD MILLION)
TABLE 51 SOUTH AFRICA E-CLINICAL SOLUTIONS MARKET, BY USER DEVICE, 2021-2030 (USD MILLION)
TABLE 52 SOUTH AFRICA E-CLINICAL SOLUTIONS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 53 SAUDI ARABIA E-CLINICAL SOLUTIONS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 54 SAUDI ARABIA E-CLINICAL SOLUTIONS MARKET, BY DELIVERY MODE, 2021-2030 (USD MILLION)
TABLE 55 SAUDI ARABIA E-CLINICAL SOLUTIONS MARKET, BY CLINICAL TRIAL PHASE, 2021-2030 (USD MILLION)
TABLE 56 SAUDI ARABIA E-CLINICAL SOLUTIONS MARKET, BY ORGANIZATION SIZE, 2021-2030 (USD MILLION)
TABLE 57 SAUDI ARABIA E-CLINICAL SOLUTIONS MARKET, BY USER DEVICE, 2021-2030 (USD MILLION)
TABLE 58 SAUDI ARABIA E-CLINICAL SOLUTIONS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 59 U.A.E. E-CLINICAL SOLUTIONS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 60 U.A.E. E-CLINICAL SOLUTIONS MARKET, BY DELIVERY MODE, 2021-2030 (USD MILLION)
TABLE 61 U.A.E. E-CLINICAL SOLUTIONS MARKET, BY CLINICAL TRIAL PHASE, 2021-2030 (USD MILLION)
TABLE 62 U.A.E. E-CLINICAL SOLUTIONS MARKET, BY ORGANIZATION SIZE, 2021-2030 (USD MILLION)
TABLE 63 U.A.E. E-CLINICAL SOLUTIONS MARKET, BY USER DEVICE, 2021-2030 (USD MILLION)
TABLE 64 U.A.E. E-CLINICAL SOLUTIONS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 65 EGYPT E-CLINICAL SOLUTIONS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 66 EGYPT E-CLINICAL SOLUTIONS MARKET, BY DELIVERY MODE, 2021-2030 (USD MILLION)
TABLE 67 EGYPT E-CLINICAL SOLUTIONS MARKET, BY CLINICAL TRIAL PHASE, 2021-2030 (USD MILLION)
TABLE 68 EGYPT E-CLINICAL SOLUTIONS MARKET, BY ORGANIZATION SIZE, 2021-2030 (USD MILLION)
TABLE 69 EGYPT E-CLINICAL SOLUTIONS MARKET, BY USER DEVICE, 2021-2030 (USD MILLION)
TABLE 70 EGYPT E-CLINICAL SOLUTIONS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 71 ISRAEL E-CLINICAL SOLUTIONS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 72 ISRAEL E-CLINICAL SOLUTIONS MARKET, BY DELIVERY MODE, 2021-2030 (USD MILLION)
TABLE 73 ISRAEL E-CLINICAL SOLUTIONS MARKET, BY CLINICAL TRIAL PHASE, 2021-2030 (USD MILLION)
TABLE 74 ISRAEL E-CLINICAL SOLUTIONS MARKET, BY ORGANIZATION SIZE, 2021-2030 (USD MILLION)
TABLE 75 ISRAEL E-CLINICAL SOLUTIONS MARKET, BY USER DEVICE, 2021-2030 (USD MILLION)
TABLE 76 ISRAEL E-CLINICAL SOLUTIONS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 77 REST OF MIDDLE EAST AND AFRICA E-CLINICAL SOLUTIONS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
図表一覧
FIGURE 1 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: SEGMENTATION
FIGURE 2 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: DATA TRIANGULATION
FIGURE 3 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: DROC ANALYSIS
FIGURE 4 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: MIDDLE EAST & AFRICA VS REGIONAL MARKET ANALYSIS
FIGURE 5 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: MARKET END USER COVERAGE GRID
FIGURE 8 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: DBMR MARKET POSITION GRID
FIGURE 9 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 MIDDLE EAST & AFRICA E- CLINICAL SOLUTIONS MARKET: SEGMENTATION
FIGURE 11 THE GROWING USAGE OF E-CLINICAL SOLUTIONS IN CLINICAL TRIALS AS WELL AS INCREASING R&D ACTIVITIES AND CORRESPONDING EXPENDITURE, IS EXPECTED TO DRIVE THE GROWTH OF THE MIDDLE EAST & AFRICA E- CLINICAL SOLUTIONS MARKET FROM 2023 TO 2030
FIGURE 12 PRODUCT SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET IN 2023 AND 2030
FIGURE 13 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET
FIGURE 14 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY PRODUCT, 2022
FIGURE 15 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY PRODUCT, 2023-2030 (USD MILLION)
FIGURE 16 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY PRODUCT, CAGR (2023-2030)
FIGURE 17 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY PRODUCT, LIFELINE CURVE
FIGURE 18 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY DELIVERY MODE, 2022
FIGURE 19 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY DELIVERY MODE, 2023-2030 (USD MILLION)
FIGURE 20 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY DELIVERY MODE, CAGR (2023-2030)
FIGURE 21 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY DELIVERY MODE, LIFELINE CURVE
FIGURE 22 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY CLINICAL TRIAL PHASE, 2022
FIGURE 23 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY CLINICAL TRIAL PHASE, 2023-2030 (USD MILLION)
FIGURE 24 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY CLINICAL TRIAL PHASE, CAGR (2023-2030)
FIGURE 25 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY CLINICAL TRIAL PHASE, LIFELINE CURVE
FIGURE 26 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY ORGANIZATION SIZE, 2022
FIGURE 27 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY ORGANIZATION SIZE, 2023-2030 (USD MILLION)
FIGURE 28 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY ORGANIZATION SIZE, CAGR (2023-2030)
FIGURE 29 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY ORGANIZATION SIZE, LIFELINE CURVE
FIGURE 30 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY USER DEVICE, 2022
FIGURE 31 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY USER DEVICE, 2023-2030 (USD MILLION)
FIGURE 32 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY USER DEVICE, CAGR (2023-2030)
FIGURE 33 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY USER DEVICE, LIFELINE CURVE
FIGURE 34 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY END USER, 2022
FIGURE 35 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY END USER, 2023-2030 (USD MILLION)
FIGURE 36 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY END USER, CAGR (2023-2030)
FIGURE 37 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY END USER, LIFELINE CURVE
FIGURE 38 MIDDLE EAST AND AFRICA E-CLINICAL SOLUTIONS MARKET: SNAPSHOT (2022)
FIGURE 39 MIDDLE EAST AND AFRICA E-CLINICAL SOLUTIONS MARKET: BY COUNTRY (2022)
FIGURE 40 MIDDLE EAST AND AFRICA E-CLINICAL SOLUTIONS MARKET: BY COUNTRY (2023 & 2030)
FIGURE 41 MIDDLE EAST AND AFRICA E-CLINICAL SOLUTIONS MARKET: BY COUNTRY (2022 & 2030)
FIGURE 42 MIDDLE EAST AND AFRICA E-CLINICAL SOLUTIONS MARKET: PRODUCT (2023-2030)
FIGURE 43 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: COMPANY SHARE 2022 (%)
調査方法
データ収集と基準年分析は、大規模なサンプル サイズのデータ収集モジュールを使用して行われます。この段階では、さまざまなソースと戦略を通じて市場情報または関連データを取得します。過去に取得したすべてのデータを事前に調査および計画することも含まれます。また、さまざまな情報ソース間で見られる情報の不一致の調査も含まれます。市場データは、市場統計モデルと一貫性モデルを使用して分析および推定されます。また、市場シェア分析と主要トレンド分析は、市場レポートの主要な成功要因です。詳細については、アナリストへの電話をリクエストするか、お問い合わせをドロップダウンしてください。
DBMR 調査チームが使用する主要な調査方法は、データ マイニング、データ変数が市場に与える影響の分析、および一次 (業界の専門家) 検証を含むデータ三角測量です。データ モデルには、ベンダー ポジショニング グリッド、市場タイムライン分析、市場概要とガイド、企業ポジショニング グリッド、特許分析、価格分析、企業市場シェア分析、測定基準、グローバルと地域、ベンダー シェア分析が含まれます。調査方法について詳しくは、お問い合わせフォームから当社の業界専門家にご相談ください。
カスタマイズ可能
Data Bridge Market Research は、高度な形成的調査のリーダーです。当社は、既存および新規のお客様に、お客様の目標に合致し、それに適したデータと分析を提供することに誇りを持っています。レポートは、対象ブランドの価格動向分析、追加国の市場理解 (国のリストをお問い合わせください)、臨床試験結果データ、文献レビュー、リファービッシュ市場および製品ベース分析を含めるようにカスタマイズできます。対象競合他社の市場分析は、技術ベースの分析から市場ポートフォリオ戦略まで分析できます。必要な競合他社のデータを、必要な形式とデータ スタイルでいくつでも追加できます。当社のアナリスト チームは、粗い生の Excel ファイル ピボット テーブル (ファクト ブック) でデータを提供したり、レポートで利用可能なデータ セットからプレゼンテーションを作成するお手伝いをしたりすることもできます。