North America Drug Safety Solutions and Pharmacovigilance Market Size, Share and Trends Analysis Report
Market Size in USD Billion
CAGR :
% 
 USD
2.04 Billion
USD
5.75 Billion
2025
2033
USD
2.04 Billion
USD
5.75 Billion
2025
2033
| 2026 –2033 | |
| USD 2.04 Billion | |
| USD 5.75 Billion | |
|
|
|
|
North America Drug Safety Solutions and Pharmacovigilance Market Segmentation, By Type (Software and Services), End Users (Biotechnology and Pharmaceuticals, Contract Research Organizations (CROS), Hospitals, KPOs/BPOs and Healthcare Providers), Distribution Channel (Direct Sales and Retail Sales) - Industry Trends and Forecast to 2033
North America Drug Safety Solutions And Pharmacovigilance Market Size
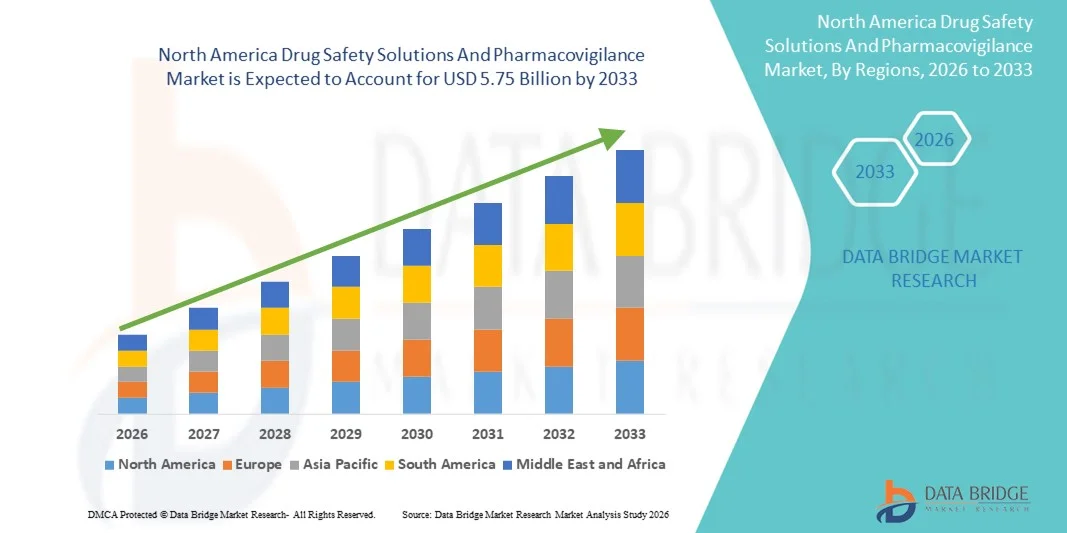
- The North America drug safety solutions and pharmacovigilance market size was valued at USD 2.04 billion in 2025 and is expected to reach USD 5.75 billion by 2033, at a CAGR of 13.85% during the forecast period
- The market growth is largely fueled by increasing regulatory compliance requirements, rising adoption of digital tools in clinical trials, and growing awareness of patient safety and risk management in the pharmaceutical industry
- Furthermore, the expanding use of real-world evidence, big data analytics, and AI-driven pharmacovigilance platforms is enabling more efficient monitoring of drug safety and adverse events. These converging factors are accelerating the uptake of Drug Safety Solutions and Pharmacovigilance solutions, thereby significantly boosting the industry's growth
North America Drug Safety Solutions And Pharmacovigilance Market Analysis
- Drug Safety Solutions and Pharmacovigilance are increasingly critical in ensuring patient safety, monitoring adverse drug reactions, and maintaining compliance with stringent regulatory standards across the pharmaceutical and biotechnology industries
- The growing demand is driven by the adoption of digital pharmacovigilance platforms, AI and machine learning for adverse event prediction, and the integration of real-world evidence into drug safety monitoring. These factors are significantly boosting the uptake of Drug Safety Solutions and Pharmacovigilance globally
- The U.S. dominated the drug safety solutions and pharmacovigilance market with the largest revenue share of approximately 42% in 2025, supported by the presence of leading pharmaceutical companies, advanced regulatory frameworks, and early adoption of AI-based pharmacovigilance solutions
- Canada is expected to be the fastest-growing region during the forecast period, registering a CAGR of 9.8%, driven by increasing healthcare digitization, supportive government policies, and rising investments in clinical trials and drug safety monitoring
- The software segment dominated the largest market revenue share of 61.8% in 2025, driven by the increasing adoption of integrated pharmacovigilance platforms for adverse event reporting, signal detection, and regulatory compliance
Report Scope and Drug Safety Solutions And Pharmacovigilance Market Segmentation
|
Attributes |
Drug Safety Solutions And Pharmacovigilance Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
|
|
Key Market Players |
• Oracle Health Sciences (U.S.) |
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
North America Drug Safety Solutions And Pharmacovigilance Market Trends
Growing Adoption of Cloud-Based and Integrated Safety Platforms
- A significant and accelerating trend in the global drug safety solutions and pharmacovigilance market is the increasing adoption of cloud-based and integrated safety platforms. These platforms enable seamless collection, processing, and reporting of adverse drug events across multiple regions and regulatory frameworks
- For instance, in June 2023, ArisGlobal launched its cloud-based safety platform, LifeSphere Safety, offering real-time adverse event reporting, automated signal detection, and compliance management across global regulatory standards. This launch reflects the shift towards centralized, scalable, and interoperable pharmacovigilance systems
- Modern pharmacovigilance platforms integrate electronic health records (EHRs), clinical trial data, and post-marketing surveillance information to provide actionable insights for risk management
- The integration of advanced analytics and machine learning algorithms allows for early detection of safety signals, reducing time-to-action for potential drug risks
- The trend is further supported by regulatory authorities emphasizing real-time and transparent reporting for patient safety, prompting companies to adopt more efficient and compliant solutions
- In addition, the scalability of cloud solutions enables pharmaceutical companies of all sizes to implement robust pharmacovigilance processes without significant infrastructure investment
North America Drug Safety Solutions And Pharmacovigilance Market Dynamics
Driver
Regulatory Pressure and Increasing Drug Development Activities
- The increasing complexity of regulatory compliance and pharmacovigilance requirements worldwide is a major driver for market growth. Regulatory authorities such as the FDA, EMA, and PMDA mandate stringent reporting of adverse drug reactions, driving demand for automated and standardized solutions
- For instance, in March 2024, IQVIA expanded its safety and pharmacovigilance services in Europe to support pharmaceutical companies in meeting EMA guidelines on periodic safety update reports (PSURs) and risk management plans (RMPs)
- Rapid growth in drug development pipelines, especially for biologics and specialty drugs, increases the need for robust post-marketing safety monitoring solutions
- Pharmaceutical companies are investing in centralized safety databases to manage data from clinical trials, post-marketing surveillance, and patient registries
- Cloud-based pharmacovigilance systems improve collaboration between global teams, ensuring timely regulatory submissions
- The growing focus on patient-centric care, safety, and risk mitigation further accelerates the adoption of comprehensive pharmacovigilance platforms
Restraint/Challenge
High Implementation Costs and Data Security Concerns
- Despite the benefits, high implementation costs and integration complexity pose challenges for market adoption, particularly for small and mid-sized pharmaceutical companies. Initial investment in advanced software, training, and process redesign can be significant
- For instance, transitioning from legacy safety systems to modern cloud-based platforms may require months of configuration and validation, temporarily slowing internal operations
- Data privacy and cybersecurity concerns also remain a critical restraint, as pharmacovigilance systems handle sensitive patient and clinical data. Breaches can lead to regulatory penalties and reputational damage
- Ensuring compliance with data protection regulations such as GDPR in Europe, HIPAA in the U.S., and other regional laws adds to operational complexity
- Continuous updates, audits, and system validation are necessary to maintain regulatory compliance and avoid potential disruptions in drug safety reporting
- Overcoming these challenges requires strategic investment in secure, scalable solutions, workforce training, and robust IT infrastructure to ensure both compliance and operational efficiency
North America Drug Safety Solutions And Pharmacovigilance Market Scope
The market is segmented on the basis of type, end user, and distribution channel.
- By Type
On the basis of type, the Drug Safety Solutions and Pharmacovigilance market is segmented into software and services. The software segment dominated the largest market revenue share of 61.8% in 2025, driven by the increasing adoption of integrated pharmacovigilance platforms for adverse event reporting, signal detection, and regulatory compliance. Pharmaceutical and biotechnology companies increasingly rely on safety software to automate case intake, data processing, and submission to regulatory authorities such as the FDA and EMA. The growing volume of clinical trial data and post-marketing surveillance activities has intensified the need for centralized safety databases. Software solutions improve operational efficiency, reduce manual errors, and ensure timely compliance with global regulations. Integration with clinical data management and electronic health record systems further supports dominance. Continuous software upgrades aligned with evolving regulatory guidelines also sustain adoption. Large enterprises prefer licensed platforms due to scalability and long-term cost efficiency. As a result, safety software remains the backbone of pharmacovigilance operations globally.
The services segment is expected to witness the fastest CAGR of 13.9% from 2026 to 2033, driven by increasing outsourcing of pharmacovigilance activities to specialized service providers. Pharmaceutical companies are increasingly outsourcing case processing, medical review, and regulatory submissions to reduce operational burden and control costs. The rise in small and mid-sized biotech firms lacking in-house safety expertise further accelerates service demand. Contract safety organizations provide flexibility and access to trained professionals. Services also support compliance across multiple geographies with varying regulatory requirements. Growing drug approvals and post-marketing commitments increase reliance on outsourced safety operations. Consequently, pharmacovigilance services are emerging as a high-growth segment globally.
- By End User
On the basis of end user, the Drug Safety Solutions and Pharmacovigilance market is segmented into biotechnology and pharmaceutical companies, contract research organizations (CROs), hospitals, KPOs/BPOs, and healthcare providers. The biotechnology and pharmaceutical companies segment dominated the largest market revenue share of 48.4% in 2025, owing to stringent regulatory requirements for drug safety monitoring throughout the product lifecycle. These companies conduct extensive clinical trials and post-marketing surveillance, generating large volumes of safety data. Mandatory adverse event reporting and risk management plans drive continuous investment in pharmacovigilance systems. Global drug launches further necessitate centralized safety platforms. Large pharma companies adopt end-to-end solutions to ensure compliance across regions. Increased focus on patient safety and risk mitigation strengthens segment dominance. Continuous pipeline expansion sustains long-term demand for safety solutions within this segment.
The CROs segment is projected to register the fastest CAGR of 14.6% from 2026 to 2033, supported by the growing trend of outsourcing clinical trials and safety monitoring activities. CROs increasingly manage pharmacovigilance operations on behalf of sponsors. Expansion of global clinical research activities fuels safety service demand. CROs provide cost-effective, scalable solutions with regulatory expertise. Smaller pharma companies prefer CROs for end-to-end safety management. Growth in biologics and specialty drugs further supports adoption. As outsourcing penetration increases, CROs emerge as the fastest-growing end-user segment.
- By Distribution Channel
On the basis of distribution channel, the Drug Safety Solutions and Pharmacovigilance market is segmented into direct sales and retail sales. The direct sales segment accounted for the largest market revenue share of 67.2% in 2025, driven by the complexity and customization requirements of pharmacovigilance solutions. Software vendors and service providers typically engage directly with pharmaceutical companies to tailor solutions according to regulatory and operational needs. Direct engagement ensures proper implementation, training, and validation. Long-term contracts and enterprise licensing models support revenue stability. Regulatory compliance requirements also favor vendor-client collaboration through direct channels. Large organizations prefer direct procurement for data security and system integration. As a result, direct sales dominate market distribution.
小売販売セグメントは、モジュール型およびクラウドベースの安全性ソリューションの利用可能性の向上に牽引され、2026年から2033年にかけて11.8%という最も高いCAGRで成長すると予想されています。小規模なバイオテクノロジー企業や医療機関は、導入が迅速な既製のプラットフォームを好みます。サブスクリプションベースの価格設定モデルは、手頃な価格を実現します。デジタルヘルスマーケットプレイスの成長は、アクセス性を高めます。小売チャネルは、新興企業の調達の複雑さを軽減します。中小企業における導入の増加は、成長を加速させます。このように、小売販売は急速に拡大する流通チャネルとなっています。
北米の医薬品安全性ソリューションおよび医薬品安全性監視市場の地域分析
- 北米は、2025年に約42%という最大の収益シェアで医薬品安全性ソリューションおよび医薬品安全性監視市場を支配しました。このリーダーシップは、世界的な製薬企業やバイオテクノロジー企業の強力な存在、米国FDAやカナダ保健省などの機関が主導する確立された規制環境、そして先進的な医薬品安全性監視技術の早期導入によって支えられています。
- この地域では、有害事象の検出、シグナル管理、規制遵守を改善するために、AIと機械学習に基づく医薬品安全性プラットフォームの導入が増加しています。
- さらに、高い研究開発費、広範な臨床試験活動、市販後薬物監視への重点の高まりが、北米全体の市場成長を強化し続けています。
米国の医薬品安全性ソリューションおよび医薬品安全性監視市場に関する洞察:
米国の医薬品安全性ソリューションおよび医薬品安全性監視市場は、2025年に北米の医薬品安全性ソリューションおよび医薬品安全性監視市場を席巻し、地域全体の収益シェアの大部分を占めました。米国市場の成長は、大手製薬会社、開発業務受託機関(CRO)、そして専門的な医薬品安全性監視サービスプロバイダーの存在によって牽引されています。医薬品承認および市販後調査に関する厳格な規制要件に加え、AIを活用した症例処理、リアルワールドエビデンス分析、クラウドベースの安全性データベースの早期導入が、需要を大幅に押し上げています。さらに、医薬品開発、生物製剤、個別化医療の複雑性の高まりにより、臨床段階から商業段階まで、堅牢な医薬品安全性監視ソリューションの必要性が高まっています。
Canada Drug Safety Solutions and Pharmacovigilance Market Insight
Canada drug safety solutions and pharmacovigilance market is expected to be the fastest-growing country in the Drug Safety Solutions and Pharmacovigilance market during the forecast period, registering a CAGR of around 9.8%. This growth is driven by increasing healthcare digitization, supportive government initiatives to enhance drug safety monitoring, and rising investments in clinical trials. The expanding presence of global pharmaceutical companies and CROs in Canada, along with growing adoption of electronic adverse event reporting systems and integrated pharmacovigilance platforms, is further supporting market expansion. In addition, closer alignment with international regulatory standards is encouraging pharmaceutical manufacturers to strengthen their drug safety operations in the country.
North America Drug Safety Solutions And Pharmacovigilance Market Share
The Drug Safety Solutions And Pharmacovigilance industry is primarily led by well-established companies, including:
• Oracle Health Sciences (U.S.)
• IQVIA (U.S.)
• Veeva Systems (U.S.)
• ArisGlobal (U.S.)
• Dassault Systèmes (France)
• Cognizant Technology Solutions (U.S.)
• Wipro (India)
• Tata Consultancy Services – TCS (India)
• Accenture (Ireland)
• Parexel International (U.S.)
• ICON plc (Ireland)
• Labcorp Drug Development (U.S.)
• Medidata Solutions (U.S.)
• Ennov (France)
• EXTEDO (Germany)
• CliniSys (U.K.)
• United BioSource Corporation (U.S.)
Latest Developments in North America Drug Safety Solutions And Pharmacovigilance Market
- In December 2023, Thermo Fisher Scientific launched CorEvidence, a cloud-based data lake platform designed to optimize pharmacovigilance case processing and safety data management by streamlining adverse event management and supporting regulatory requirements for post-authorization safety studies
- In July 2024, Oracle introduced new AI-enhanced capabilities in its Argus and Safety One Intake solutions to help life science organizations manage increasing adverse event workloads, automate safety case processing, enhance global regulatory compliance, and improve data privacy and reporting efficiency. This update reflects the shift toward AI-driven drug safety platforms that support pharmaceutical companies and CROs in meeting evolving regulatory demands
- In April 2024, Qinecsa Solutions acquired Danish-based Insife ApS, strengthening its position as a leading provider of digital pharmacovigilance solutions by combining Qinecsa’s expertise with Insife’s HALOPV platform, enhancing global drug safety data management and pharmacovigilance technology offerings
- 2024年11月、Veeva SystemsはSafetyConnectを立ち上げ、Vault Safetyプラットフォームを拡張しました。これにより、AI支援によるトリアージと多言語処理により、世界中の関連会社間で統一された安全性ケース管理が可能になり、グローバルな医薬品安全性監視ケースの処理が改善され、レポートワークフローが加速されます。
- 2025年3月、アリスグローバルはIBMとの戦略的提携を発表し、IBMのAI機能をアリスグローバルライフスフィアと統合して医薬品安全性監視ケースの処理を加速し、安全性データ分析を強化しました。これは、より効率的な安全性監視とリスク管理のためのAIの採用の増加を反映しています。
SKU-
世界初のマーケットインテリジェンスクラウドに関するレポートにオンラインでアクセスする
- インタラクティブなデータ分析ダッシュボード
- 成長の可能性が高い機会のための企業分析ダッシュボード
- カスタマイズとクエリのためのリサーチアナリストアクセス
- インタラクティブなダッシュボードによる競合分析
- 最新ニュース、更新情報、トレンド分析
- 包括的な競合追跡のためのベンチマーク分析のパワーを活用
調査方法
データ収集と基準年分析は、大規模なサンプル サイズのデータ収集モジュールを使用して行われます。この段階では、さまざまなソースと戦略を通じて市場情報または関連データを取得します。過去に取得したすべてのデータを事前に調査および計画することも含まれます。また、さまざまな情報ソース間で見られる情報の不一致の調査も含まれます。市場データは、市場統計モデルと一貫性モデルを使用して分析および推定されます。また、市場シェア分析と主要トレンド分析は、市場レポートの主要な成功要因です。詳細については、アナリストへの電話をリクエストするか、お問い合わせをドロップダウンしてください。
DBMR 調査チームが使用する主要な調査方法は、データ マイニング、データ変数が市場に与える影響の分析、および一次 (業界の専門家) 検証を含むデータ三角測量です。データ モデルには、ベンダー ポジショニング グリッド、市場タイムライン分析、市場概要とガイド、企業ポジショニング グリッド、特許分析、価格分析、企業市場シェア分析、測定基準、グローバルと地域、ベンダー シェア分析が含まれます。調査方法について詳しくは、お問い合わせフォームから当社の業界専門家にご相談ください。
カスタマイズ可能
Data Bridge Market Research は、高度な形成的調査のリーダーです。当社は、既存および新規のお客様に、お客様の目標に合致し、それに適したデータと分析を提供することに誇りを持っています。レポートは、対象ブランドの価格動向分析、追加国の市場理解 (国のリストをお問い合わせください)、臨床試験結果データ、文献レビュー、リファービッシュ市場および製品ベース分析を含めるようにカスタマイズできます。対象競合他社の市場分析は、技術ベースの分析から市場ポートフォリオ戦略まで分析できます。必要な競合他社のデータを、必要な形式とデータ スタイルでいくつでも追加できます。当社のアナリスト チームは、粗い生の Excel ファイル ピボット テーブル (ファクト ブック) でデータを提供したり、レポートで利用可能なデータ セットからプレゼンテーションを作成するお手伝いをしたりすることもできます。