Global Addisons Disease Drugs Market
시장 규모 (USD 10억)
연평균 성장률 :
% 
 USD
674.61 Million
USD
1,499.52 Million
2024
2032
USD
674.61 Million
USD
1,499.52 Million
2024
2032
| 2025 –2032 | |
| USD 674.61 Million | |
| USD 1,499.52 Million | |
|
|
|
|
Global Addison's Disease Drugs Market Segmentation, By Diagnosis (Laboratory Tests and Image Testing), Treatment (Oral Corticosteroid and Corticosteroid Injections), End User (Hospitals and Clinics, Diagnostic Laboratories, and Others) – Industry Trends and Forecast to 2032
Addison's Disease Drug Market Size
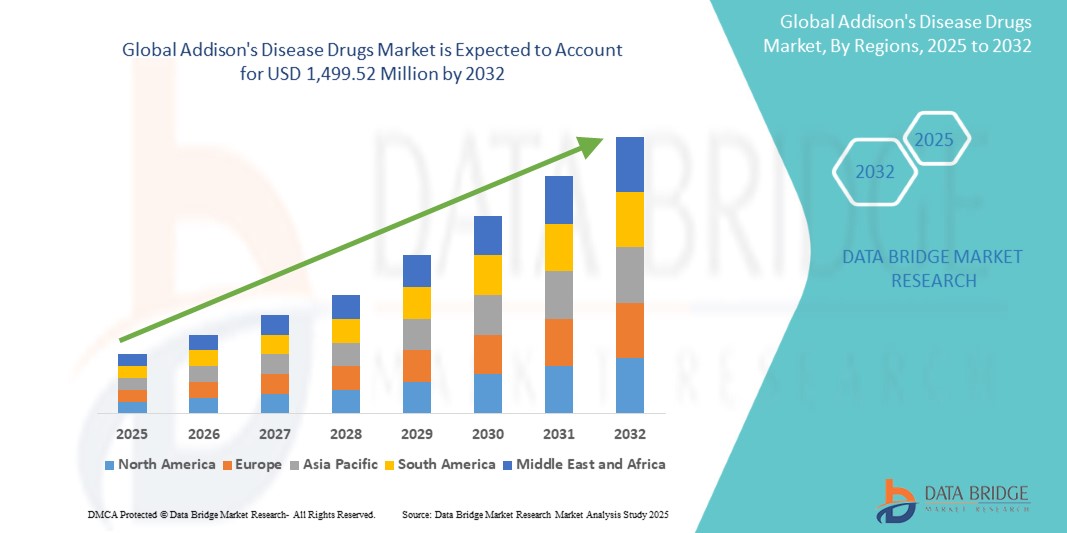
- The global Addison's Disease Drug Market was valued at USD 1.02 billion in 2024 and is expected to reach USD 1.96 billion by 2032.
- During the forecast period of 2025 to 2032, the market is projected to grow at a CAGR of 8.4%, primarily driven by increasing prevalence of adrenal insufficiency, improved diagnostic rates, and growing awareness of hormone replacement therapies.
- This growth is fueled by factors such as advancements in endocrinology, improved patient compliance with long-term treatment regimens, and ongoing research into novel corticosteroid formulations.
Addison's Disease Drug Market Analysis
- The global Addison's Disease Drug Market is anticipated to gain notable traction during the forecast period, with a CAGR of 8.4% from 2025 to 2032.
- Addison’s disease—characterized by chronic adrenal insufficiency—is often associated with autoimmune destruction of the adrenal cortex, infections, or secondary adrenal insufficiency due to pituitary disorders.
- The market is being supported by the increasing availability of synthetic corticosteroids and mineralocorticoids, enhanced focus on patient-centric dosing solutions, and strategic collaborations among pharmaceutical companies.
- Rising healthcare investments, early diagnosis via ACTH stimulation testing, and expanded access to hormone therapies in low- and middle-income countries are expected to further drive market growth
Report Scope andGastrointestinal Small Molecule API Market Segmentation
|
Attributes |
Medication-Assisted Treatment (MAT) KeyMarket Insights |
|
Segments Covered |
•By Drug: lucocorticoids (e.g., hydrocortisone), Mineralocorticoids (e.g., fludrocortisone) and Others |
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include import export analysis, production capacity overview, production consumption analysis, price trend analysis, climate change scenario, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Addison's Disease Drug Market Trends
“Shift Toward Hormone Replacement and Precision Endocrine Therapies”
- A prominent trend in the Addison's Disease Drug Market is the shift toward more targeted and patient-centric hormone replacement regimens. Traditional glucocorticoid and mineralocorticoid therapies are being refined with newer delivery methods and dosing algorithms to better mimic natural circadian rhythms and reduce long-term complications.
- For instance, modified-release hydrocortisone formulations are gaining traction for offering more physiologic cortisol exposure and improved quality of life in patients.
- The integration of endocrine monitoring tools, digital health apps, and AI-driven dosage personalization is enhancing treatment precision and adherence.Additionally, research is progressing on subcutaneous and continuous delivery systems, such as pumps and implants, to reduce adrenal crises and improve patient outcomes.
Addison's Disease Drug Market Dynamics
Driver
“Rising Prevalence of Autoimmune Adrenal Insufficiency and Associated Comorbidities”
- The growing incidence of autoimmune disorders is significantly contributing to primary adrenal insufficiency cases worldwide, particularly in developed regions.
- For instance: According to the European Society of Endocrinology (2023), autoimmune Addison’s disease accounts for over 70% of primary adrenal insufficiency cases in Western countries.
- The chronic and life-threatening nature of the disease necessitates lifelong hormone therapy and robust patient management, driving demand for effective and safe drug options.
“Regulatory Advancements and Orphan Drug Support for Rare Endocrine Disorders”
- Favorable regulatory policies and incentives for rare disease drug development are accelerating innovation and market access for Addison's disease treatments.
- For example: In 2022, the FDA granted orphan drug designation and priority review status to several modified-release corticosteroid therapies designed for adrenal insufficiency, supporting the development of more physiologic replacement strategies.
Opportunity
“Pipeline Growth and Partnerships in Endocrine Replacement and Adjunctive Therapies”
- The Addison's Disease Drug Market is experiencing robust growth in pipeline activity, driven by increased R&D investments and strategic alliances.
- For example: Companies are developing next-generation hormone formulations, stress-dose therapy protocols, and adjunctive agents that aim to optimize metabolic control and reduce morbidity.
-
Collaborations between academic institutions and pharmaceutical firms are accelerating translational research in adrenal biology and immunomodulation.
-
As awareness increases around the impact of adrenal crises and unmet treatment needs, opportunities for novel delivery platforms and patient-specific regimens are expanding rapidly.
Restraint/Challenge
“High Costs and Individual Variability in Hormone Therapy Response”
- Despite advancements, the market is constrained by the high cost of newer drug formulations and delivery systems.Access to optimized endocrine care remains limited in low-resource settings, leading to disparities in treatment adherence and outcomes.Moreover, variability in pharmacokinetics and patient response, especially during stress or illness, poses ongoing challenges to consistent disease control.
- For example: Modified-release hydrocortisone therapies, while beneficial, are significantly more expensive than conventional formulations and may not be covered under standard insurance in many regions, limiting widespread adoption.
Gastrointestinal Small Molecule API Market Scope
The market is segmented on the basis of type, drug class, , route of administration, end-users, and distribution channel.
|
Segmentation |
Sub-Segmentation |
|
Drug Class |
|
|
Route of Administration |
|
|
End-Users |
|
|
Distribution Channel |
|
Global Addison’s Disease Drug Market Regional Analysis
“North America is the Dominant Region in the Addison’s Disease Drug Market”
- North America dominates the global Addison’s Disease Drug market owing to its advanced healthcare infrastructure, strong awareness of rare endocrine disorders, and increasing access to orphan drugs.
- The United States holds the largest market share in this region due to early diagnosis rates, strong support for rare disease treatment, and the presence of prominent pharmaceutical companies engaged in endocrine therapy.
- The market is supported by favorable reimbursement policies, regulatory incentives from the FDA (such as Orphan Drug Designation), and a robust pipeline of hormone replacement therapies.
- Increasing initiatives to raise awareness about adrenal insufficiency and ongoing clinical trials of extended-release corticosteroids are contributing to market expansion.
- Strategic collaborations among research institutions and pharmaceutical firms, along with high adoption of innovative delivery mechanisms such as subcutaneous implants and once-daily oral formulations, are fueling innovation.
“Asia-Pacific is Projected to Register the Highest Growth Rate”
- The Asia-Pacific region is anticipated to grow at the fastest pace in the Addison’s Disease Drug market due to rising healthcare awareness, increasing diagnosis rates, and growing adoption of rare disease therapies.
- Countries like India, China, Japan, and Australia are key contributors, owing to expanding healthcare infrastructure, increased patient access to endocrinologists, and efforts to address underserved rare disease populations.
- National health initiatives and rare disease policies are improving accessibility and reimbursement frameworks for Addison’s disease medications across the region.
- Japan, with its strong pharmaceutical industry and patient-centric healthcare system, continues to play a vital role in advancing treatment options.
- Expanding local manufacturing, supportive government funding, and partnerships between regional companies and multinational pharma firms are also accelerating the market in Asia-Pacific.
Addison’s Disease Drug Market Share
The competitive landscape provides insights into the major market players, including their company profiles, financial strength, R&D investments, product offerings, global reach, production capabilities, and strategic initiatives.
The Major Market Leaders Operating in the Market Include:
- Bristol Myers Squibb Company
- Takeda Pharmaceutical Company Limited
- Merck & Co., Inc.
- Novartis AG
- Pfizer Inc.
- Hikma Pharmaceuticals PLC
- Teva Pharmaceutical Industries Ltd.
- Cipla Limited
- Endo International plc
- GlaxoSmithKline plc
- Sun Pharmaceutical Industries Ltd.
- Sanofi S.A.
- AbbVie Inc.
- Amneal Pharmaceuticals LLC
- Zydus Lifesciences
- Mylan N.V.
- Novo Nordisk A/S
- Bio-Techne Corporation
- Ferring Pharmaceutical
- Recordati Rare Diseases Inc.
Latest Developments in Global Addison’s Disease Drug Market
- In February 2023, the European Medicines Agency (EMA) issued guidelines aimed at improving the clinical development of therapies for rare endocrine disorders, including Addison’s disease. These included considerations for innovative delivery routes and long-acting corticosteroids.
- The industry is witnessing a growing trend toward the development of personalized hormone replacement therapies, emphasis on adrenal-specific biomarkers, and increased use of digital platforms for patient monitoring. Additionally, there is a strong focus on improving drug adherence and reducing side effects through modified-release and dual-release formulations.
SKU-
세계 최초의 시장 정보 클라우드 보고서에 온라인으로 접속하세요
- 대화형 데이터 분석 대시보드
- 높은 성장 잠재력 기회를 위한 회사 분석 대시보드
- 사용자 정의 및 질의를 위한 리서치 분석가 액세스
- 대화형 대시보드를 통한 경쟁자 분석
- 최신 뉴스, 업데이트 및 추세 분석
- 포괄적인 경쟁자 추적을 위한 벤치마크 분석의 힘 활용
연구 방법론
데이터 수집 및 기준 연도 분석은 대규모 샘플 크기의 데이터 수집 모듈을 사용하여 수행됩니다. 이 단계에는 다양한 소스와 전략을 통해 시장 정보 또는 관련 데이터를 얻는 것이 포함됩니다. 여기에는 과거에 수집한 모든 데이터를 미리 검토하고 계획하는 것이 포함됩니다. 또한 다양한 정보 소스에서 발견되는 정보 불일치를 검토하는 것도 포함됩니다. 시장 데이터는 시장 통계 및 일관된 모델을 사용하여 분석하고 추정합니다. 또한 시장 점유율 분석 및 주요 추세 분석은 시장 보고서의 주요 성공 요인입니다. 자세한 내용은 분석가에게 전화를 요청하거나 문의 사항을 드롭하세요.
DBMR 연구팀에서 사용하는 주요 연구 방법론은 데이터 마이닝, 시장에 대한 데이터 변수의 영향 분석 및 주요(산업 전문가) 검증을 포함하는 데이터 삼각 측량입니다. 데이터 모델에는 공급업체 포지셔닝 그리드, 시장 타임라인 분석, 시장 개요 및 가이드, 회사 포지셔닝 그리드, 특허 분석, 가격 분석, 회사 시장 점유율 분석, 측정 기준, 글로벌 대 지역 및 공급업체 점유율 분석이 포함됩니다. 연구 방법론에 대해 자세히 알아보려면 문의를 통해 업계 전문가에게 문의하세요.
사용자 정의 가능
Data Bridge Market Research는 고급 형성 연구 분야의 선두 주자입니다. 저희는 기존 및 신규 고객에게 목표에 맞는 데이터와 분석을 제공하는 데 자부심을 느낍니다. 보고서는 추가 국가에 대한 시장 이해(국가 목록 요청), 임상 시험 결과 데이터, 문헌 검토, 재생 시장 및 제품 기반 분석을 포함하도록 사용자 정의할 수 있습니다. 기술 기반 분석에서 시장 포트폴리오 전략에 이르기까지 타겟 경쟁업체의 시장 분석을 분석할 수 있습니다. 귀하가 원하는 형식과 데이터 스타일로 필요한 만큼 많은 경쟁자를 추가할 수 있습니다. 저희 분석가 팀은 또한 원시 엑셀 파일 피벗 테이블(팩트북)로 데이터를 제공하거나 보고서에서 사용 가능한 데이터 세트에서 프레젠테이션을 만드는 데 도움을 줄 수 있습니다.