Global Biomaterial Testing Equipment Market
시장 규모 (USD 10억)
연평균 성장률 :
% 
 USD
3.77 Billion
USD
4.97 Billion
2024
2032
USD
3.77 Billion
USD
4.97 Billion
2024
2032
| 2025 –2032 | |
| USD 3.77 Billion | |
| USD 4.97 Billion | |
|
|
|
|
Global Biomaterial Testing Equipment Market Segmentation, By Type (Contact Type, and Contactless Type), Application (Cardiovascular, Orthopedic, Dental, and Ophthalmology) Product Type (Universal Testing Machines, Dynamic Mechanical Analyzers, Rheometers, Hardness Testers, and Thermogravimetric Analyzers), End User (Healthcare, Pharmaceuticals, Research Institutions, Manufacturers, and Government Agencies) – Industry Trends and Forecast to 2032
Biomaterial Testing Equipment Market Analysis
The biomaterial testing equipment market has experienced significant growth due to the increasing demand for advanced materials in the medical, healthcare, and pharmaceutical industries. These testing systems are crucial for assessing the physical, mechanical, and chemical properties of biomaterials, ensuring their safety and efficacy for medical devices, implants, and other healthcare applications. The market is driven by the rising prevalence of chronic diseases, the growing aging population, and the continuous advancements in biomaterials such as polymers, ceramics, and composites. Recent developments in the industry include innovations in automated testing systems, real-time data analysis, and the integration of artificial intelligence to enhance precision and efficiency. In addition, the increasing focus on regulatory compliance and the need for high-quality biomaterials to meet safety standards are also contributing to market growth. The rising adoption of biomaterial testing equipment is expected to continue as manufacturers strive to meet stringent requirements and improve patient outcomes.
Biomaterial Testing Equipment Market Size
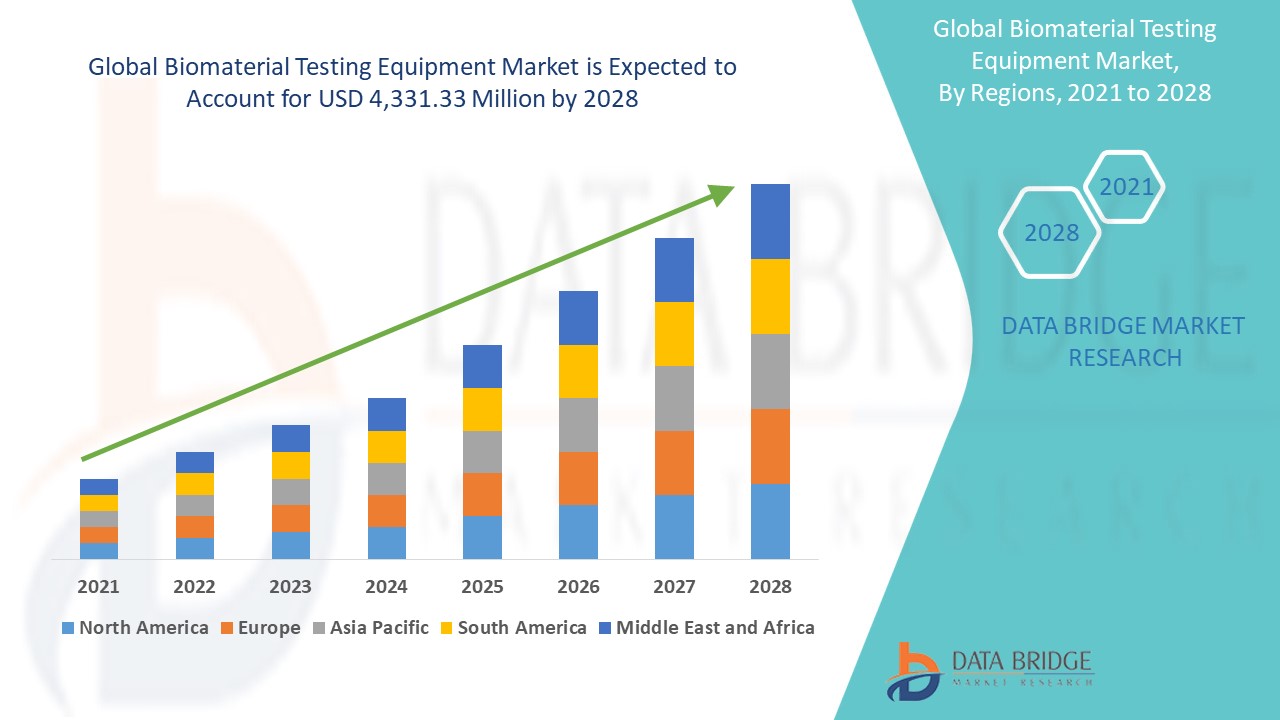
The global biomaterial testing equipment market size was valued at USD 3.77 billion in 2024 and is projected to reach USD 4.97 billion by 2032, with a CAGR of 3.51% during the forecast period of 2025 to 2032. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Biomaterial Testing Equipment Market Trends
“Increasing Demand for Advanced Medical Materials”
The biomaterial testing equipment market is witnessing notable trends driven by innovations in testing technologies and increasing demand for advanced medical materials. As healthcare and biomedical industries grow, there is a rising need for precise testing of biomaterials used in implants, prosthetics, and other medical devices. A key innovation is the integration of artificial intelligence and machine learning into testing systems, allowing for real-time data analysis and improved accuracy. One prominent trend is the increasing adoption of automated testing equipment, which enhances efficiency, reduces human error, and ensures consistent quality in material testing. As regulatory standards for biomaterials become more stringent, the demand for advanced testing equipment is expected to rise, driving market expansion and ensuring product safety and reliability.
Report Scope and Biomaterial Testing Equipment Market Segmentation
|
Attributes |
Biomaterial Testing Equipment Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E., South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America. |
|
Key Market Players |
Shimadzu Corporation (Japan), Intertek Group plc (U.K.), ZwickRoell Pvt. Ltd. (Germany), CellScale (Canada), Applied Test Systems (U.S.), AMETEK, Inc. (U.S.), Rheolution Inc. (Canada), MTS Systems (U.S.), Bio Materials (U.S.), ADMET, Inc. (U.S.), Presto Group (India), Fluke Corporation (U.S.), Illinois Tool Works Inc. (U.S.), Rigel Medical (U.K.), Nordson Corporation (U.S.), Buehler (U.S.), ReScience (Finland), Environics (Finland) |
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Biomaterial Testing Equipment Market Definition
Biomaterial testing equipment refers to specialized instruments and systems used to evaluate the physical, chemical, and mechanical properties of biomaterials. These materials, which are typically used in medical devices, implants, prosthetics, and tissue engineering, must undergo rigorous testing to ensure their safety, durability, and compatibility with biological systems. Biomaterial testing equipment includes devices such as universal testing machines, hardness testers, rheometers, and dynamic mechanical analyzers. These tools help assess characteristics such as tensile strength, elasticity, fatigue resistance, and biocompatibility, ensuring that biomaterials meet the required standards for use in healthcare applications.
Biomaterial Testing Equipment Market Dynamics
Drivers
- Increasing Demand for Advanced Medical Devices
The growing demand for high-performance biomaterials in medical devices, implants, and prosthetics is a significant driver of the biomaterial testing equipment market. As medical technologies advance, the materials used in these devices must meet stringent safety, durability, and biocompatibility standards. Precise testing equipment is essential to ensure that biomaterials, such as polymers, ceramics, and metals, are suitable for long-term implantation or use within the human body. This includes testing for properties such as mechanical strength, corrosion resistance, and tissue compatibility. The increasing reliance on innovative medical solutions further emphasizes the need for advanced testing systems to guarantee material safety and efficacy.
- Innovations in Testing Technologies
The growing demand for high-performance biomaterials in medical devices, implants, and prosthetics is a significant driver of the biomaterial testing equipment market. As medical technologies advance, the materials used in these devices must meet stringent safety, durability, and biocompatibility standards. Precise testing equipment is essential to ensure that biomaterials, such as polymers, ceramics, and metals, are suitable for long-term implantation or use within the human body. This includes testing for properties such as mechanical strength, corrosion resistance, and tissue compatibility. The increasing reliance on innovative medical solutions further emphasizes the need for advanced testing systems to guarantee material safety and efficacy.
Opportunities
- Advancements in Personalized Medicine
The growing focus on personalized and regenerative medicine is creating significant opportunities in the biomaterial testing equipment market. As treatments become more tailored to individual patients, there is an increasing need for customized biomaterials that are specifically designed to meet the unique biological requirements of each patient. This shift drives the demand for specialized testing equipment to assess the properties and compatibility of these tailored biomaterials. Advanced testing solutions are required to ensure the safety, durability, and efficacy of these materials in medical applications, such as implants and tissue regeneration. As personalized medicine continues to evolve, the market for biomaterial testing equipment is expected to grow substantially.
- Growth in Biotechnology and Tissue Engineering
The rise of bioprinting, tissue engineering, and other advanced biotechnologies is driving the demand for specialized testing of novel biomaterials, offering significant growth opportunities in the biomaterial testing equipment market. As these innovative technologies advance, they require new types of biomaterials with specific properties, such as biocompatibility, strength, and flexibility. This creates a need for precise and specialized testing equipment to ensure the safety, effectiveness, and quality of these materials. In addition, as research and development in tissue engineering and bioprinting expand, there will be an increasing demand for testing equipment that can accommodate the unique requirements of these emerging biomaterials, further fueling market growth.
Restraints/Challenges
- Limited Skilled Workforce
The specialized nature of biomaterial testing poses a significant challenge due to the need for a highly skilled workforce capable of operating advanced equipment and interpreting complex data. The testing of biomaterials requires expertise in various fields, including materials science, engineering, and biotechnology. In many regions, there is a shortage of professionals with the necessary training and qualifications to handle sophisticated testing equipment and analyze intricate results. This skills gap can delay the adoption of advanced biomaterial testing technologies, hinder efficient testing processes, and potentially increase costs for companies and research institutions, ultimately slowing market growth.
- High Initial Investment Costs
Advanced biomaterial testing equipment typically requires substantial upfront investment, both in capital and infrastructure, which presents a significant barrier for smaller companies or emerging markets. The high costs associated with acquiring state-of-the-art testing systems, along with the need for specialized facilities to house and operate them, can be prohibitive for organizations with limited financial resources. This restraint can limit the ability of smaller players or those in developing regions to access advanced testing technologies, potentially hindering their participation in the market. Consequently, the high initial investment required can slow down the adoption and growth of biomaterial testing equipment in these segments.
This market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Biomaterial Testing Equipment Market Scope
The market is segmented on the basis of type, application, product type, and end user. The growth amongst these segments will help you analyse meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Type
- Contact Type
- Contactless Type
Application
- Cardiovascular
- Orthopedic
- Dental
- Ophthalmology
Product Type
- Universal Testing Machines
- Dynamic Mechanical Analyzers
- Rheometers
- Hardness Testers
- Thermogravimetric Analyzers
End User
- Healthcare
- Pharmaceuticals
- Research Institutions
- Manufacturers
- Government Agencies
Biomaterial Testing Equipment Market Regional Analysis
The market is analysed and market size insights and trends are provided by country, type, application, product type, and end user as referenced above.
The countries covered in the market report are U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E., South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
North America dominates biomaterial testing equipment market due to the high prevalence of cardiovascular diseases, a growing aging population, and a well-established healthcare infrastructure. The region’s advanced medical research and healthcare systems further drive the demand for precise biomaterial testing. In addition, increasing focus on improving patient outcomes and regulatory standards in healthcare contribute to North America's market dominance.
Asia-Pacific is projected to experience the fastest growth in the biomaterial testing equipment market from 2025 to 2032, driven by a rapidly growing population and the ongoing development of healthcare infrastructure. The region's increasing healthcare investments and demand for advanced medical devices contribute to this growth. In addition, the rising awareness of quality healthcare and regulatory standards further accelerates the adoption of biomaterial testing technologies in the region.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points such as down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Biomaterial Testing Equipment Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
Biomaterial Testing Equipment Market Leaders Operating in the Market Are:
- Shimadzu Corporation (Japan)
- Intertek Group plc (U.K.)
- ZwickRoell Pvt. Ltd. (Germany)
- CellScale (Canada)
- Applied Test Systems (U.S.)
- AMETEK, Inc. (U.S.)
- Rheolution Inc. (Canada)
- MTS Systems (U.S.)
- Bio Materials (U.S.)
- ADMET, Inc. (U.S.)
- Presto Group (India)
- Fluke Corporation (U.S.)
- Illinois Tool Works Inc. (U.S.)
- Rigel Medical (U.K.)
- Nordson Corporation (U.S.)
- Buehler (U.S.)
- ReScience (Finland)
- Environics (Finland)
Latest Developments in Biomaterial Testing Equipment Market
- In May 2024, Osstem Implant expanded its market presence by acquiring Implacil de Bortoli, Brazil’s third-largest dental implant company. This acquisition strengthens Osstem Implant’s position in the Latin American dental implant market. The move reflects the company’s strategy to enhance its global footprint and cater to the growing demand for dental solutions in the region
- In October 2023, Bolt Threads, Inc., a biomaterials platform, reached an agreement with the special purpose acquisition company (SPAC) Golden Arrow Merger Corp. This deal paved the way for Bolt Threads to go public. The partnership reflects the company’s growth strategy to expand its reach in the biomaterials industry and access new investment opportunities
- In August 2023, CJ Biomaterials signed a supply agreement with Riman to provide eco-friendly packaging materials for Riman’s products. This collaboration aims to promote sustainable practices in packaging, aligning with the growing demand for environmentally responsible solutions. The agreement highlights CJ Biomaterials' commitment to advancing green technologies and supporting sustainability in various industries
SKU-
세계 최초의 시장 정보 클라우드 보고서에 온라인으로 접속하세요
- 대화형 데이터 분석 대시보드
- 높은 성장 잠재력 기회를 위한 회사 분석 대시보드
- 사용자 정의 및 질의를 위한 리서치 분석가 액세스
- 대화형 대시보드를 통한 경쟁자 분석
- 최신 뉴스, 업데이트 및 추세 분석
- 포괄적인 경쟁자 추적을 위한 벤치마크 분석의 힘 활용
연구 방법론
데이터 수집 및 기준 연도 분석은 대규모 샘플 크기의 데이터 수집 모듈을 사용하여 수행됩니다. 이 단계에는 다양한 소스와 전략을 통해 시장 정보 또는 관련 데이터를 얻는 것이 포함됩니다. 여기에는 과거에 수집한 모든 데이터를 미리 검토하고 계획하는 것이 포함됩니다. 또한 다양한 정보 소스에서 발견되는 정보 불일치를 검토하는 것도 포함됩니다. 시장 데이터는 시장 통계 및 일관된 모델을 사용하여 분석하고 추정합니다. 또한 시장 점유율 분석 및 주요 추세 분석은 시장 보고서의 주요 성공 요인입니다. 자세한 내용은 분석가에게 전화를 요청하거나 문의 사항을 드롭하세요.
DBMR 연구팀에서 사용하는 주요 연구 방법론은 데이터 마이닝, 시장에 대한 데이터 변수의 영향 분석 및 주요(산업 전문가) 검증을 포함하는 데이터 삼각 측량입니다. 데이터 모델에는 공급업체 포지셔닝 그리드, 시장 타임라인 분석, 시장 개요 및 가이드, 회사 포지셔닝 그리드, 특허 분석, 가격 분석, 회사 시장 점유율 분석, 측정 기준, 글로벌 대 지역 및 공급업체 점유율 분석이 포함됩니다. 연구 방법론에 대해 자세히 알아보려면 문의를 통해 업계 전문가에게 문의하세요.
사용자 정의 가능
Data Bridge Market Research는 고급 형성 연구 분야의 선두 주자입니다. 저희는 기존 및 신규 고객에게 목표에 맞는 데이터와 분석을 제공하는 데 자부심을 느낍니다. 보고서는 추가 국가에 대한 시장 이해(국가 목록 요청), 임상 시험 결과 데이터, 문헌 검토, 재생 시장 및 제품 기반 분석을 포함하도록 사용자 정의할 수 있습니다. 기술 기반 분석에서 시장 포트폴리오 전략에 이르기까지 타겟 경쟁업체의 시장 분석을 분석할 수 있습니다. 귀하가 원하는 형식과 데이터 스타일로 필요한 만큼 많은 경쟁자를 추가할 수 있습니다. 저희 분석가 팀은 또한 원시 엑셀 파일 피벗 테이블(팩트북)로 데이터를 제공하거나 보고서에서 사용 가능한 데이터 세트에서 프레젠테이션을 만드는 데 도움을 줄 수 있습니다.