Global Ocular Pain Intravitreal Treatment Market
시장 규모 (USD 10억)
연평균 성장률 :
% 
 USD
79.21 Million
USD
146.61 Million
2024
2032
USD
79.21 Million
USD
146.61 Million
2024
2032
| 2025 –2032 | |
| USD 79.21 Million | |
| USD 146.61 Million | |
|
|
|
|
Global Ocular Pain Intravitreal Treatment Market Segmentation, By Drug Type (Anti-inflammatory agents, Analgesics, Anti-infectives, Biologics, and Sustained-release implants), Indication (Post-operative ocular pain, Intraocular inflammation, Endophthalmitis, Retinal vascular disease–associated pain, and neuropathic ocular pain), Delivery Route (Intravitreal injection, Periocular, Intracameral, and subretinal), End User (Hospitals, Ambulatory Surgical Centers (ASCs), Specialty eye clinics, and Pharmacies) - Industry Trends and Forecast to 2032
Ocular Pain Intravitreal Treatment Market Size
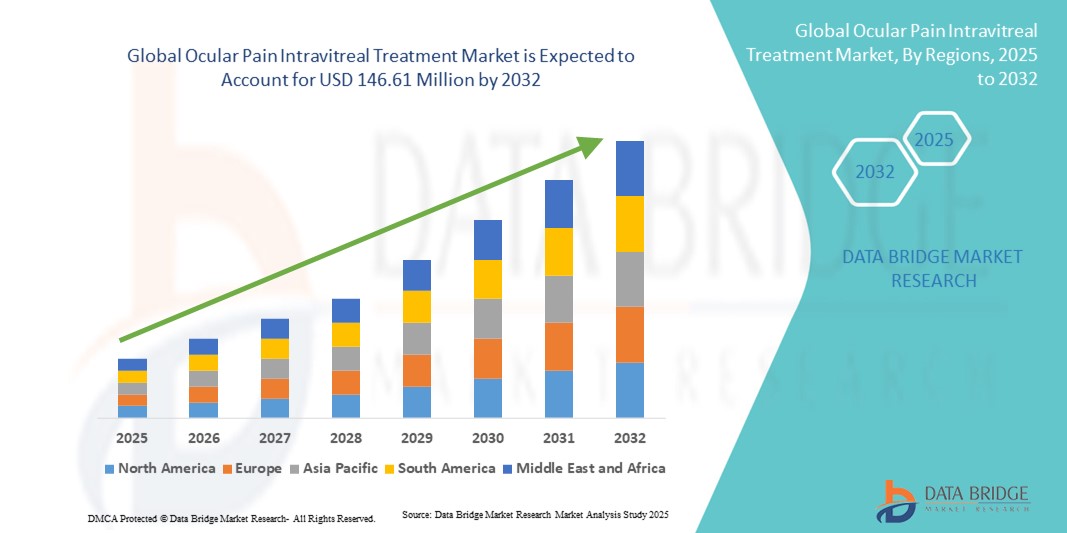
- The global ocular pain intravitreal treatment market size was valued at USD 79.21 million in 2024 and is expected to reach USD 146.61 million by 2032, at a CAGR of 8.00% during the forecast period
- The market growth is largely driven by the rising prevalence of ophthalmic disorders such as uveitis, endophthalmitis, and post-surgical complications, which often result in significant ocular pain requiring intravitreal interventions
- Furthermore, the increasing adoption of sustained-release implants, targeted anti-inflammatory agents, and innovative analgesic formulations is positioning intravitreal delivery as a preferred modality for effective ocular pain management. These clinical and technological advancements are accelerating treatment uptake, thereby fueling the market’s expansion globally
Ocular Pain Intravitreal Treatment Market Analysis
- Ocular pain intravitreal treatments, including anti-inflammatory agents, analgesics, anti-infectives, biologics, and sustained-release implants, are emerging as critical therapeutic options for managing pain caused by uveitis, endophthalmitis, retinal vascular disease, and post-operative complications, due to their targeted delivery and longer therapeutic effect
- The rising demand for these treatments is primarily fueled by the increasing prevalence of ophthalmic disorders, growing surgical volumes in ophthalmology, and the need for effective, long-acting solutions that minimize repeated interventions and enhance patient outcomes
- North America dominated the ocular pain intravitreal treatment market with the largest revenue share of 42.5% in 2024, supported by advanced healthcare infrastructure, a robust pipeline of ocular drugs and implants, and high adoption of intravitreal therapies across hospitals and specialty eye clinics in the U.S.
- Asia-Pacific is expected to be the fastest growing region in the ocular pain intravitreal treatment market during the forecast period, driven by a rising burden of ophthalmic diseases, expanding access to advanced eye care, and increased investments in ophthalmology-focused research and infrastructure
- The anti-inflammatory agents segment dominated the ocular pain intravitreal treatment market with a share of 43% in 2024, attributed to the widespread use of corticosteroids and steroid implants as the gold standard for reducing ocular inflammation and associated pain
Report Scope and Ocular Pain Intravitreal Treatment Market Segmentation
|
Attributes |
Ocular Pain Intravitreal Treatment Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Ocular Pain Intravitreal Treatment Market Trends
Advancements in Sustained-Release Implants and Targeted Therapies
- A significant and accelerating trend in the global ocular pain intravitreal treatment market is the rapid adoption of sustained-release implants and biologics designed to provide long-lasting relief from inflammation and pain, reducing the burden of repeated intravitreal injections
- For instance, the Ozurdex (dexamethasone intravitreal implant) has gained strong clinical acceptance for managing ocular pain associated with uveitis and macular edema, demonstrating how extended-release technologies are reshaping treatment protocols
- Newer research pipelines are focusing on targeted drug delivery systems that enhance therapeutic precision, improve ocular tissue penetration, and minimize systemic side effects, further driving innovation in intravitreal pain therapies
- For instance, investigational biologics and depot-based analgesic formulations are being developed to offer more effective and sustained relief for patients with chronic ocular pain or recurrent inflammatory conditions
- The integration of advanced polymer technology and nanocarrier systems into intravitreal therapies facilitates controlled drug release, thereby offering more predictable treatment outcomes and fewer intervention visits
- This trend towards longer-acting, precision-targeted, and patient-friendly intravitreal treatments is redefining ophthalmic pain management, creating new benchmarks for efficacy and convenience
Ocular Pain Intravitreal Treatment Market Dynamics
Driver
Rising Burden of Ophthalmic Disorders and Surgical Interventions
- The increasing prevalence of ophthalmic conditions such as uveitis, endophthalmitis, and retinal vascular diseases, combined with rising rates of cataract and vitrectomy surgeries, is significantly driving the demand for intravitreal treatments for ocular pain
- For instance, in 2024, the American Academy of Ophthalmology highlighted a surge in post-surgical ocular inflammation cases, emphasizing the role of intravitreal corticosteroids and implants in effective pain management
- As patient awareness of advanced ocular therapies grows, intravitreal treatments are being recognized as essential tools for long-term control of ocular inflammation, delivering sustained relief and preserving vision outcomes
- Furthermore, expanding healthcare infrastructure and reimbursement support in developed markets are propelling the adoption of intravitreal pain management solutions across hospitals and specialty eye clinics
- The proven ability of intravitreal therapies to deliver targeted, localized drug concentrations makes them a preferred choice over systemic medications, reinforcing their dominance in ophthalmic pain treatment strategies.
- The shift toward personalized medicine and ongoing innovations in ophthalmology pipelines further strengthen the growth trajectory of intravitreal pain management solutions globally
Restraint/Challenge
Invasive Delivery Method and Regulatory Compliance Hurdles
- Concerns surrounding the invasive nature of intravitreal injections, including risks of infection, retinal detachment, and patient discomfort, pose a significant challenge to widespread acceptance of ocular pain intravitreal treatments
- For instance, multiple reports of endophthalmitis associated with intravitreal procedures have led to heightened caution among both patients and providers, limiting uptake in certain regions
- Addressing these safety concerns through innovations in minimally invasive delivery systems, improved injection devices, and enhanced physician training is crucial to strengthen patient confidence
- In addition, stringent regulatory pathways for intravitreal therapies, requiring extensive clinical trials and safety evaluations, can delay product approvals and market entry for novel pain management drugs and implants
- The relatively high cost of advanced implants and biologics compared to traditional topical or systemic pain therapies creates barriers to adoption, especially in cost-sensitive healthcare markets
- Overcoming these challenges through affordable pricing models, streamlined regulatory strategies, and enhanced patient education on treatment benefits will be vital for sustained growth in the intravitreal pain treatment market
Ocular Pain Intravitreal Treatment Market Scope
The market is segmented on the basis of drug type, indication, delivery route, and end user.
- By Drug Type
On the basis of drug type, the ocular pain intravitreal treatment market is segmented into anti-inflammatory agents, analgesics, anti-infectives, biologics, and sustained-release implants. Anti-inflammatory agents dominated the market with the largest revenue share in 2024, accounting for 43%. Corticosteroids such as dexamethasone and triamcinolone are widely used for managing inflammation and ocular pain linked to conditions such as uveitis and post-surgical complications. Their proven efficacy, well-established clinical guidelines, and availability in both intravitreal injection and implant forms make them the preferred first-line therapy. Hospitals and ophthalmologists also favor anti-inflammatory drugs due to their ability to deliver rapid relief, reduce recurrence rates, and maintain long-term therapeutic outcomes. In addition, the increasing availability of sustained-release steroid implants further enhances this segment’s dominance.
The biologics segment is anticipated to witness the fastest growth rate from 2025 to 2032, fueled by the expanding pipeline of monoclonal antibodies and gene therapy-based approaches targeting underlying inflammatory pathways. Biologics offer the potential to address refractory or chronic ocular pain cases where traditional corticosteroids are less effective or associated with side effects. Moreover, the growing number of clinical trials, regulatory approvals, and increased investment in ophthalmic biologics are strengthening adoption. Rising awareness of targeted therapies, coupled with the push toward personalized medicine, positions biologics as the fastest-expanding category.
- By Indication
On the basis of indication, the market is segmented into post-operative ocular pain, intraocular inflammation, endophthalmitis, retinal vascular disease–associated pain, and neuropathic ocular pain. Post-operative ocular pain dominated the market in 2024, supported by the rising global volume of cataract and vitrectomy surgeries. These procedures frequently lead to inflammation and pain, making intravitreal corticosteroids and analgesics critical for effective recovery. Hospitals and ambulatory surgical centers rely heavily on intravitreal therapies to minimize complications, shorten recovery periods, and improve patient comfort. The segment’s growth is also supported by favorable reimbursement policies in developed markets and increased adoption of sustained-release implants, which reduce the need for multiple post-surgical interventions.
Neuropathic ocular pain is expected to be the fastest-growing segment during the forecast period, driven by increasing recognition of neuropathic eye conditions and unmet needs in chronic ocular pain management. Traditional treatments often fail to adequately address nerve-related pain, creating opportunities for novel analgesics, biologics, and advanced delivery systems. Research into targeted pathways, such as ion channel modulators and neuroprotective biologics, is expanding rapidly. As awareness grows among ophthalmologists and patients, coupled with advancements in diagnostic capabilities, neuropathic ocular pain therapies are projected to experience the sharpest rise in demand.
- By Delivery Route
On the basis of delivery route, the market is segmented into intravitreal injection, periocular, intracameral, and subretinal. Intravitreal injection dominated the market in 2024 with the largest share, as it remains the gold standard for delivering high drug concentrations directly to the posterior segment of the eye. Its widespread use in both hospitals and specialty clinics for corticosteroids, antibiotics, and biologics underscores its central role in ocular pain management. Physicians prefer this route due to its precision, rapid onset of action, and ability to bypass systemic exposure, thereby minimizing side effects. The well-established safety profile, supported by clinical data and decades of experience, further secures its leading position.
The subretinal route is anticipated to be the fastest-growing delivery mode over the forecast period, driven by its increasing application in advanced therapies such as gene therapy and regenerative medicine. Though still in the early stages of adoption, subretinal delivery enables highly targeted treatment for retinal disorders associated with pain. Rising investment in R&D, clinical trial activity, and technological innovation in micro-surgical techniques are accelerating this segment’s growth. As more therapies gain regulatory approval, subretinal delivery is expected to carve out a strong growth trajectory in the coming years.
- By End User
On the basis of end user, the market is segmented into hospitals, ambulatory surgical centers (ASCs), specialty eye clinics, and pharmacies. Hospitals dominated the market in 2024 with a revenue share of 55%, supported by their advanced infrastructure, ability to handle complex surgical interventions, and access to the latest intravitreal therapies. Hospitals also manage a higher patient load for post-operative and acute conditions requiring immediate pain relief, making them the primary setting for ocular pain treatment. The presence of skilled ophthalmologists, coupled with reimbursement frameworks in developed countries, further strengthens this segment’s leadership.
Ambulatory Surgical Centers (ASCs) are expected to witness the fastest growth from 2025 to 2032, driven by the global shift toward cost-effective and outpatient ophthalmic procedures. ASCs provide quicker turnaround, reduced treatment costs, and greater patient convenience compared to hospitals. The rising demand for cataract and retina surgeries in outpatient settings, along with expanding investments in equipping ASCs with advanced intravitreal drug delivery capabilities, is fueling rapid adoption. This trend is especially prominent in North America and Asia-Pacific, where healthcare systems are focusing on efficiency and patient-centered care.
Ocular Pain Intravitreal Treatment Market Regional Analysis
- North America dominated the ocular pain intravitreal treatment market with the largest revenue share of 42.5% in 2024, supported by advanced healthcare infrastructure, a robust pipeline of ocular drugs and implants, and high adoption of intravitreal therapies across hospitals and specialty eye clinics in the U.S.
- Patients and providers in the region highly value the effectiveness of intravitreal injections, implants, and biologics in reducing ocular pain while maintaining vision outcomes
- This widespread adoption is further supported by favorable reimbursement policies, robust clinical research infrastructure, and the presence of leading pharmaceutical and biotech companies actively developing new ophthalmic therapies
U.S. Ocular Pain Intravitreal Treatment Market Insight
The U.S. ocular pain intravitreal treatment market captured the largest revenue share of 79% in 2024 within North America, fueled by the high prevalence of retinal diseases, glaucoma, and post-surgical ocular complications. Patients are increasingly prioritizing intravitreal therapies for effective pain relief and better visual outcomes. The strong presence of leading pharmaceutical companies, combined with rapid adoption of biologics and sustained-release implants, further propels market growth. Moreover, supportive reimbursement frameworks and extensive use of advanced delivery systems, such as sustained-release depots, are significantly contributing to the market’s expansion.
Europe Ocular Pain Intravitreal Treatment Market Insight
The Europe ocular pain intravitreal treatment market is projected to expand at a substantial CAGR throughout the forecast period, primarily driven by rising surgical interventions for cataract and retinal conditions. The growing burden of ocular inflammation and endophthalmitis is fostering the adoption of intravitreal injections. European healthcare providers are also drawn to innovative biologics and anti-inflammatory agents offering durable relief. The region is experiencing strong adoption across hospitals, specialty eye clinics, and ambulatory surgical centers, with intravitreal treatments increasingly integrated into routine ophthalmic care.
U.K. Ocular Pain Intravitreal Treatment Market Insight
The U.K. ocular pain intravitreal treatment market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by increasing incidences of intraocular inflammation and heightened patient awareness of advanced ophthalmic care. In addition, concerns over post-operative ocular pain and vision preservation are encouraging patients and providers to adopt intravitreal biologics and analgesic therapies. The country’s robust healthcare infrastructure, coupled with high clinical trial activity, is expected to continue to stimulate market growth.
Germany Ocular Pain Intravitreal Treatment Market Insight
The Germany ocular pain intravitreal treatment market is expected to expand at a considerable CAGR during the forecast period, fueled by growing demand for biologics and sustained-release implants to manage ocular pain effectively. Germany’s advanced healthcare infrastructure and emphasis on innovation support the integration of new therapies into clinical practice. The presence of leading academic research institutions, along with strong adoption in both public and private healthcare settings, is promoting the use of intravitreal solutions tailored for long-term pain management.
Asia-Pacific Ocular Pain Intravitreal Treatment Market Insight
The Asia-Pacific ocular pain intravitreal treatment market is poised to grow at the fastest CAGR of 23% during the forecast period of 2025 to 2032, driven by increasing surgical volumes, aging populations, and rising incidence of diabetic retinopathy and retinal vascular diseases. The region’s growing adoption of advanced biologics and innovative delivery routes, supported by government-led healthcare modernization, is fueling rapid uptake. Furthermore, as APAC emerges as a clinical trial and manufacturing hub for ophthalmic drugs, the affordability and accessibility of intravitreal therapies are expanding to a wider patient base.
Japan Ocular Pain Intravitreal Treatment Market Insight
The Japan ocular pain intravitreal treatment market is gaining momentum due to the country’s advanced healthcare system, rising elderly population, and growing burden of retinal and inflammatory eye diseases. The Japanese market places a strong emphasis on innovative biologics and drug-delivery systems, driving the adoption of sustained-release implants. The integration of intravitreal treatments with broader ophthalmic care protocols, including diabetic eye disease management, is fueling growth. Moreover, Japan’s technological advancements and patient demand for minimally invasive therapies are expected to further accelerate adoption.
India Ocular Pain Intravitreal Treatment Market Insight
The India ocular pain intravitreal treatment market accounted for the largest market revenue share in Asia-Pacific in 2024, attributed to the country’s high prevalence of post-operative ocular pain, infections, and diabetic retinopathy-related complications. India stands as one of the largest markets for ophthalmic surgeries, and intravitreal therapies are becoming increasingly popular across hospitals and specialty clinics. The push towards expanding ophthalmology infrastructure and the availability of cost-effective intravitreal injections, alongside strong domestic pharmaceutical manufacturing, are key factors propelling the market in India.
Ocular Pain Intravitreal Treatment Market Share
The Ocular Pain Intravitreal Treatment industry is primarily led by well-established companies, including:
- AbbVie Inc. (U.S.)
- Genentech, Inc. (U.S.)
- Regeneron Pharmaceuticals Inc. (U.S.)
- Novartis AG (Switzerland)
- Alimera Sciences (U.S.)
- Bausch + Lomb (U.S.)
- Santen Pharmaceutical Co., Ltd. (Japan)
- EyePoint Pharmaceuticals, Inc. (U.S.)
- Clearside Biomedical. (U.S.)
- Alcon Inc. (Switzerland)
- Apellis Pharmaceuticals, Inc. (U.S.)
- Opthea Limited (Australia)
- Astellas Pharma Inc. (U.S.)
- Oxurion NV (Belgium)
- Ocular Therapeutix, Inc. (U.S.)
- Kala Pharmaceuticals, Inc. (U.S.)
- Bayer AG (Germany)
- SIFI S.p.A. (Italy)
- Dompé (Italy)
What are the Recent Developments in Global Ocular Pain Intravitreal Treatment Market?
- In July 2025, positive Phase 2a trial results for PER-001, a novel slow-release intravitreal implant targeting endothelin receptors, were published. The implant demonstrated improvements in vision, retinal ischemia, and structural parameters in patients with glaucoma and diabetic retinopathy, highlighting its potential to address pain and ischemia associated with progressive ocular disease
- In April 2025, ANI Pharmaceuticals announced FDA approval expanding the label of ILUVIEN® to include chronic non-infectious uveitis of the posterior segment. The label extension enhances the clinical utility of the implant in ocular pain management and provides physicians with a durable intravitreal option for patients suffering from painful inflammatory eye disorders
- In March 2025, the U.S. FDA approved the fluocinolone acetonide intravitreal implant ILUVIEN® for the treatment of chronic non-infectious uveitis affecting the posterior segment of the eye (NIU-PS), expanding its therapeutic reach beyond diabetic macular edema. This approval underscores growing recognition of intravitreal implants as effective long-term solutions for inflammatory ocular pain and related complications
- In February 2025, Genentech’s Susvimo® received U.S. FDA approval as a continuous delivery platform of ranibizumab for the treatment of diabetic macular edema (DME). This approval provides patients with fewer intravitreal treatments than standard injections, reducing pain burden and improving compliance in long-term retinal disease management
- In October 2024, Okyo Pharma dosed the first patient in a Phase 2 trial of OK-101, a novel therapy designed for neuropathic corneal pain (NCP). As a chronic ocular pain condition with limited treatment options, this milestone trial represents a significant step toward developing non-opioid, non-systemic solutions to relieve persistent corneal pain
SKU-
세계 최초의 시장 정보 클라우드 보고서에 온라인으로 접속하세요
- 대화형 데이터 분석 대시보드
- 높은 성장 잠재력 기회를 위한 회사 분석 대시보드
- 사용자 정의 및 질의를 위한 리서치 분석가 액세스
- 대화형 대시보드를 통한 경쟁자 분석
- 최신 뉴스, 업데이트 및 추세 분석
- 포괄적인 경쟁자 추적을 위한 벤치마크 분석의 힘 활용
연구 방법론
데이터 수집 및 기준 연도 분석은 대규모 샘플 크기의 데이터 수집 모듈을 사용하여 수행됩니다. 이 단계에는 다양한 소스와 전략을 통해 시장 정보 또는 관련 데이터를 얻는 것이 포함됩니다. 여기에는 과거에 수집한 모든 데이터를 미리 검토하고 계획하는 것이 포함됩니다. 또한 다양한 정보 소스에서 발견되는 정보 불일치를 검토하는 것도 포함됩니다. 시장 데이터는 시장 통계 및 일관된 모델을 사용하여 분석하고 추정합니다. 또한 시장 점유율 분석 및 주요 추세 분석은 시장 보고서의 주요 성공 요인입니다. 자세한 내용은 분석가에게 전화를 요청하거나 문의 사항을 드롭하세요.
DBMR 연구팀에서 사용하는 주요 연구 방법론은 데이터 마이닝, 시장에 대한 데이터 변수의 영향 분석 및 주요(산업 전문가) 검증을 포함하는 데이터 삼각 측량입니다. 데이터 모델에는 공급업체 포지셔닝 그리드, 시장 타임라인 분석, 시장 개요 및 가이드, 회사 포지셔닝 그리드, 특허 분석, 가격 분석, 회사 시장 점유율 분석, 측정 기준, 글로벌 대 지역 및 공급업체 점유율 분석이 포함됩니다. 연구 방법론에 대해 자세히 알아보려면 문의를 통해 업계 전문가에게 문의하세요.
사용자 정의 가능
Data Bridge Market Research는 고급 형성 연구 분야의 선두 주자입니다. 저희는 기존 및 신규 고객에게 목표에 맞는 데이터와 분석을 제공하는 데 자부심을 느낍니다. 보고서는 추가 국가에 대한 시장 이해(국가 목록 요청), 임상 시험 결과 데이터, 문헌 검토, 재생 시장 및 제품 기반 분석을 포함하도록 사용자 정의할 수 있습니다. 기술 기반 분석에서 시장 포트폴리오 전략에 이르기까지 타겟 경쟁업체의 시장 분석을 분석할 수 있습니다. 귀하가 원하는 형식과 데이터 스타일로 필요한 만큼 많은 경쟁자를 추가할 수 있습니다. 저희 분석가 팀은 또한 원시 엑셀 파일 피벗 테이블(팩트북)로 데이터를 제공하거나 보고서에서 사용 가능한 데이터 세트에서 프레젠테이션을 만드는 데 도움을 줄 수 있습니다.