Global Pi3k Inhibitor Drug Class Market
시장 규모 (USD 10억)
연평균 성장률 :
% 
 USD
3.13 Billion
USD
5.66 Billion
2025
2033
USD
3.13 Billion
USD
5.66 Billion
2025
2033
| 2026 –2033 | |
| USD 3.13 Billion | |
| USD 5.66 Billion | |
|
|
|
|
Global PI3K Inhibitor Drug Class Market Segmentation, By Type (Idelalisib, Copanlisib, Duvelisib, Alpelisib, Umbralisib, and Others), By Indication (Breast Cancer, Chronic Lymphocytic Leukemia, Follicular Lymphoma, Non-Hodgkin Lymphoma, and Others)- Industry Trends and Forecast to 2033
PI3K Inhibitor Drug Class Market Size
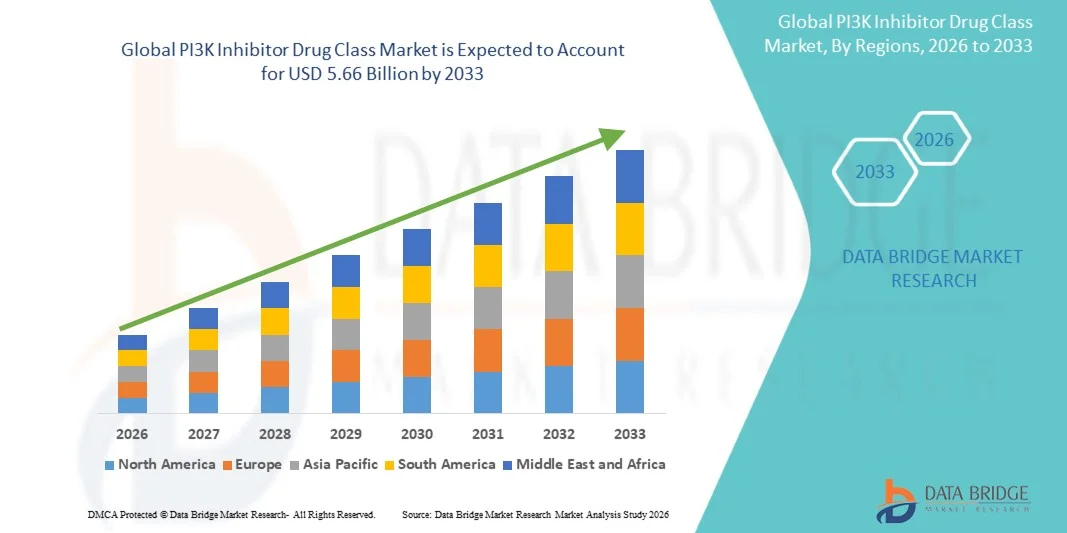
- The global PI3K Inhibitor Drug Class market size was valued at USD 3.13 billion in 2025 and is expected to reach USD 5.66 billion by 2033, at a CAGR of 7.69 % during the forecast period
- The market growth is largely fueled by the increasing adoption of targeted cancer therapies and ongoing advancements in PI3K inhibitor drug development, leading to improved treatment options for various hematologic malignancies and solid tumors
- Furthermore, rising demand for personalized oncology treatments, growing prevalence of cancers driven by PI3K pathway mutations, and continuous clinical research supporting combination therapies are establishing PI3K inhibitors as key components of modern cancer treatment protocols. These converging factors are accelerating the uptake of PI3K inhibitor drug class solutions, thereby significantly boosting overall market growth
PI3K Inhibitor Drug Class Market Analysis
- PI3K inhibitor drugs, which target the PI3K/AKT/mTOR signaling pathway involved in cell growth and survival, are increasingly vital components of modern oncology treatment due to their role in managing various hematologic malignancies and solid tumors
- The escalating demand for PI3K inhibitors is primarily fueled by rising cancer prevalence, growing adoption of targeted therapies, and ongoing clinical research supporting combination therapies and personalized treatment approaches
- North America dominated the PI3K inhibitor drug class market with the largest revenue share of approximately 41.8% in 2025, supported by advanced healthcare infrastructure, strong oncology research, high adoption of innovative cancer therapies, and presence of major pharmaceutical companies, with the U.S. accounting for a major portion of regional demand
- Asia-Pacific is expected to be the fastest-growing region in the PI3K inhibitor drug class market during the forecast period, registering a CAGR of driven by rising cancer awareness, increasing healthcare access, growing clinical trial activities, and expanding adoption of targeted oncology therapies in emerging economies
- The Breast Cancer segment dominated the market with the largest revenue share of 42.7% in 2025, driven by increasing incidence of breast cancer globally and the growing adoption of targeted therapies
Report Scope and PI3K Inhibitor Drug Class Market Segmentation
|
Attributes |
PI3K Inhibitor Drug Class Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
PI3K Inhibitor Drug Class Market Trends
“Rising Adoption of Targeted Therapies and Combination Regimens”
- A significant and accelerating trend in the global PI3K inhibitor drug class market is the increasing adoption of targeted therapies and combination regimens, especially in oncology treatment
- This trend is driven by the growing need for personalized and precision medicine, where PI3K inhibitors are combined with other targeted drugs or immunotherapies to improve patient outcomes and reduce resistance
- For instance, PI3K inhibitors are being increasingly used in combination with BTK inhibitors and monoclonal antibodies in hematologic cancers like CLL and follicular lymphoma, enhancing efficacy and reducing relapse rates
- The trend is further supported by increasing clinical evidence and ongoing research demonstrating improved survival outcomes in specific patient groups
- Furthermore, the development of newer PI3K inhibitors with improved safety profiles is encouraging wider adoption in various cancer indications
- Overall, this shift towards precision medicine and combination therapies is driving the global PI3K inhibitor drug class market growth
PI3K Inhibitor Drug Class Market Dynamics
Driver
“Growing Incidence of Cancer and Rising Demand for Precision Oncology”
- The increasing incidence of cancer globally, especially hematologic malignancies and breast cancer, is a major driver for the PI3K inhibitor drug class market
- Rising awareness about targeted therapies and improved diagnostic tools are enabling early detection and personalized treatment planning
- For instance, the increasing prevalence of breast cancer and lymphoma is pushing the adoption of PI3K inhibitors in clinical practice, especially in advanced and refractory cases
- In addition, the growing demand for precision oncology and biomarker-based treatment selection is increasing the use of PI3K inhibitors, as these drugs offer improved efficacy in genetically defined patient populations
- Higher healthcare spending and better access to oncology care in developed and emerging markets are further supporting market growth
- Overall, the rising cancer burden and precision treatment approach are expected to fuel the PI3K inhibitor drug class market during the forecast period
Restraint/Challenge
“Safety Concerns and Strict Regulatory Environment”
- A major restraint for the PI3K inhibitor drug class market is the safety profile associated with PI3K inhibitors, including risks of infections, liver toxicity, and immune-related adverse effects
- These safety concerns have led to stricter regulatory requirements and limited label expansions in some regions
- For instance, several PI3K inhibitors have received boxed warnings due to serious side effects, restricting their broader use in the market
- Moreover, high treatment costs and competition from alternative therapies such as BTK inhibitors, CAR-T, and other targeted agents may hinder market growth
- Lack of reimbursement in certain countries and limited access to specialized oncology care also pose challenges
- To overcome these restraints, manufacturers are focusing on improved safety, patient monitoring, and developing next-generation PI3K inhibitors with better tolerability
PI3K Inhibitor Drug Class Market Scope
The market is segmented on the basis of type, indication, and application.
• By Type
On the basis of type, the Global PI3K Inhibitor Drug Class market is segmented into Idelalisib, Copanlisib, Duvelisib, Alpelisib, Umbralisib, and Others. The Idelalisib segment dominated the market with the largest revenue share of 38.5% in 2025, driven by its strong established use in treating relapsed chronic lymphocytic leukemia (CLL) and follicular lymphoma. Idelalisib’s well-established clinical efficacy, favorable safety profile in selected patients, and broad adoption among oncologists contribute to its leading position. The segment benefits from strong market penetration in North America and Europe due to high awareness and reimbursement. In addition, Idelalisib’s long-standing presence has led to better real-world evidence and physician confidence, maintaining its dominance. It also enjoys steady demand due to its use in combination therapies and as a second-line treatment option. This segment is supported by ongoing clinical research, which reinforces its market position. Overall, Idelalisib remains the most preferred PI3K inhibitor in established treatment pathways, ensuring its continued dominance through the forecast period.
The Alpelisib segment is expected to witness the fastest CAGR of 22.4% from 2026 to 2033, driven by its rising adoption in targeted breast cancer therapies. Alpelisib’s strong performance in PIK3CA-mutated breast cancer patients is encouraging wider clinical use. Increasing breast cancer incidence and improved genetic testing for PIK3CA mutations are supporting its growth. The segment also benefits from higher patient awareness and improved access to targeted therapy. Rapid expansion in emerging markets, along with favorable pricing strategies, further boosts Alpelisib adoption. In addition, growing partnerships and clinical trials exploring combination therapies are expected to propel growth. Overall, Alpelisib is projected to grow fastest due to its expanding indication base and high unmet need in targeted breast cancer treatment.
• By Indication
On the basis of indication, the Global PI3K Inhibitor Drug Class market is segmented into Breast Cancer, Chronic Lymphocytic Leukemia, Follicular Lymphoma, Non-Hodgkin Lymphoma, and Others. The Breast Cancer segment dominated the market with the largest revenue share of 42.7% in 2025, driven by increasing incidence of breast cancer globally and the growing adoption of targeted therapies. The availability of PI3K inhibitors specifically for PIK3CA-mutated breast cancer patients has significantly increased the segment’s share. Rising awareness about genetic testing and personalized medicine has further strengthened this segment. The segment is also supported by increasing healthcare spending, improved diagnostic facilities, and strong physician preference for targeted therapy. Rapid expansion in emerging markets is contributing to increased patient access. In addition, continuous clinical research and improved reimbursement policies support segment growth. The segment benefits from strong demand in both developed and developing countries, leading to higher adoption rates. Overall, breast cancer remains the largest indication due to its high prevalence and need for targeted treatment.
The Chronic Lymphocytic Leukemia (CLL) segment is expected to witness the fastest CAGR of 20.1% from 2026 to 2033, driven by rising prevalence of CLL and increasing use of PI3K inhibitors as second-line treatment. Improved diagnostic capabilities and growing patient awareness support the segment’s growth. The increasing adoption of combination therapies and better access to advanced oncology treatments in emerging economies further boost demand. Ongoing clinical trials focusing on novel PI3K inhibitor combinations are expected to support growth. In addition, expanding healthcare infrastructure and reimbursement support in key regions contribute to the segment’s rapid expansion. Overall, CLL is expected to grow fastest due to rising disease burden and improved treatment options.
PI3K Inhibitor Drug Class Market Regional Analysis
- North America dominated the PI3K inhibitor drug class market with the largest revenue share of approximately 41.8% in 2025, supported by advanced healthcare infrastructure, strong oncology research, high adoption of innovative cancer therapies, and presence of major pharmaceutical companies. The U.S. accounted for a major portion of regional demand due to high treatment access, advanced diagnostic facilities, and favorable reimbursement policies
- The increasing number of clinical trials and approvals for PI3K inhibitors in hematologic cancers and breast cancer is further strengthening the market position in the region
- In addition, strong investment in oncology R&D and the presence of leading drug developers are accelerating the development and commercialization of novel PI3K inhibitors
U.S. PI3K Inhibitor Drug Class Market Insight
The U.S. PI3K inhibitor drug class market captured the largest revenue share in North America in 2025, driven by a growing prevalence of cancer and increasing demand for targeted therapies. The country has a well-established oncology treatment ecosystem and high access to innovative therapies. Strong clinical adoption, advanced diagnostic capabilities, and supportive reimbursement policies are contributing to market expansion. Moreover, ongoing research and development activities for new PI3K inhibitors and combination therapies are expected to sustain market growth during the forecast period.
Europe PI3K Inhibitor Drug Class Market Insight
The Europe PI3K inhibitor drug class market is projected to expand at a steady CAGR throughout the forecast period, driven by increasing incidence of cancer, improving healthcare infrastructure, and rising adoption of targeted therapies. Strong government initiatives for cancer treatment and early diagnosis are supporting market growth. In addition, growing clinical trial activities and the presence of leading pharmaceutical companies are fueling market expansion across major European countries.
U.K. PI3K Inhibitor Drug Class Market Insight
The U.K. PI3K inhibitor drug class market is anticipated to grow at a noteworthy CAGR during the forecast period due to rising cancer prevalence and increasing adoption of precision oncology. The country’s strong healthcare system and high research activities in oncology support the growth of targeted therapies. Furthermore, favorable reimbursement policies and early adoption of novel therapies are expected to drive market growth.
Germany PI3K Inhibitor Drug Class Market Insight
The Germany PI3K inhibitor drug class market is expected to expand at a considerable CAGR during the forecast period, fueled by increasing cancer incidence, strong healthcare infrastructure, and growing focus on advanced treatment options. Germany’s well-developed medical research ecosystem and high patient access to innovative therapies support the adoption of PI3K inhibitors. In addition, ongoing clinical trials and the presence of major pharmaceutical companies are driving market growth.
Asia-Pacific PI3K Inhibitor Drug Class Market Insight
The Asia-Pacific PI3K inhibitor drug class market is poised to grow at the fastest CAGR during the forecast period, driven by rising cancer awareness, increasing healthcare access, growing clinical trial activities, and expanding adoption of targeted oncology therapies in emerging economies. The region is witnessing rapid improvements in healthcare infrastructure, growing number of oncology centers, and rising investment in cancer treatment. As a result, the demand for PI3K inhibitors is expected to increase significantly in countries such as China, Japan, India, and South Korea.
Japan PI3K Inhibitor Drug Class Market Insight
The Japan PI3K inhibitor drug class market is gaining momentum due to strong government support for cancer research and increasing adoption of targeted therapies. Japan’s aging population and high prevalence of cancer are driving demand for advanced oncology treatments. Furthermore, ongoing clinical trials and improved access to novel therapies are expected to support market growth.
China PI3K Inhibitor Drug Class Market Insight
The China PI3K inhibitor drug class market accounted for the largest market revenue share in Asia-Pacific in 2025, attributed to increasing healthcare access, rising cancer incidence, and strong government focus on oncology treatment. The country has been rapidly expanding oncology infrastructure and increasing clinical trial activities. In addition, growing adoption of targeted therapies and improving reimbursement policies are supporting market growth in China.
PI3K Inhibitor Drug Class Market Share
The PI3K Inhibitor Drug Class industry is primarily led by well-established companies, including:
- Gilead Sciences (U.S.)
- Secura Bio (U.S.)
- Novartis (Switzerland)
- Sanofi (France)
- Bristol-Myers Squibb (U.S.)
- AstraZeneca (U.K.)
- Pfizer (U.S.)
- Takeda (Japan)
- Roche (Switzerland)
- Merck & Co. (U.S.)
- Eli Lilly (U.S.)
- AbbVie (U.S.)
- Bayer (Germany)
- GlaxoSmithKline (U.K.)
- Daiichi Sankyo (Japan)
- Johnson & Johnson (U.S.)
- Amgen (U.S.)
- BeiGene (China)
- Cytokinetics (U.S.)
- Incyte (U.S.)
Latest Developments in Global PI3K Inhibitor Drug Class Market
- In March 2023, Leniolisib (JOENJA), an oral PI3Kδ inhibitor, received its first regulatory approval for the treatment of activated PI3Kδ syndrome (APDS) in adult and pediatric patients aged 12 years and older, marking a significant expansion of the PI3K inhibitor class into non-oncology indications and highlighting the pathway’s broader therapeutic potential beyond cancer treatment
- In December 2023, Roche announced positive Phase III INAVO120 study results showing that inavolisib in combination with palbociclib and fulvestrant significantly improved progression-free survival in patients with PIK3CA-mutated, hormone receptor-positive, HER2-negative advanced breast cancer, underscoring clinical validation of next-generation PI3K inhibitors in solid tumors and strengthening the development trajectory of this drug class
- In May 2024, the U.S. Food and Drug Administration (FDA) granted Breakthrough Therapy Designation to Roche’s PI3Kα inhibitor inavolisib (in combination with palbociclib and fulvestrant) for PIK3CA-mutated, hormone receptor-positive, HER2-negative advanced breast cancer, recognising substantial clinical benefit over existing therapies and accelerating regulatory processes for this regimen
- In May 2024, the U.S. FDA accepted Roche’s New Drug Application for inavolisib and granted Priority Review for its use in PIK3CA-mutated, hormone receptor-positive, HER2-negative advanced breast cancer, signalling a faster regulatory evaluation aimed at bringing this potential first-line PI3K inhibitor therapy to patients more quickly
- In July 2024, Chugai Pharmaceutical Co., Ltd. announced it had in-licensed exclusive development and marketing rights in Japan for Roche’s PI3K inhibitor inavolisib for PIK3CA-mutated breast cancer, marking a strategic regional expansion of the drug’s development footprint in Asia and strengthening collaboration within the PI3K inhibitor market
- In October 2024, the U.S. FDA approved inavolisib (brand name Itovebi) in combination with palbociclib and fulvestrant for the treatment of adults with endocrine-resistant, PIK3CA-mutated, hormone receptor (HR)-positive, HER2-negative locally advanced or metastatic breast cancer, offering a new targeted therapy option for a common breast cancer subtype and expanding the clinical relevance of PI3K inhibitors
- In May 2025, the European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) issued a positive opinion recommending approval of Roche’s Itovebi (inavolisib) for PIK3CA-mutated, hormone receptor-positive, HER2-negative advanced breast cancer, supporting anticipated EU marketing authorization and further global expansion of this targeted PI3K inhibitor regimen
- In July 2025, the European Commission officially approved Roche’s Itovebi (inavolisib) regimen for adults with PIK3CA-mutated, ER-positive, HER2-negative advanced breast cancer, marking a significant regulatory milestone for the PI3K inhibitor class and enhancing treatment options for patients across multiple regions
SKU-
세계 최초의 시장 정보 클라우드 보고서에 온라인으로 접속하세요
- 대화형 데이터 분석 대시보드
- 높은 성장 잠재력 기회를 위한 회사 분석 대시보드
- 사용자 정의 및 질의를 위한 리서치 분석가 액세스
- 대화형 대시보드를 통한 경쟁자 분석
- 최신 뉴스, 업데이트 및 추세 분석
- 포괄적인 경쟁자 추적을 위한 벤치마크 분석의 힘 활용
연구 방법론
데이터 수집 및 기준 연도 분석은 대규모 샘플 크기의 데이터 수집 모듈을 사용하여 수행됩니다. 이 단계에는 다양한 소스와 전략을 통해 시장 정보 또는 관련 데이터를 얻는 것이 포함됩니다. 여기에는 과거에 수집한 모든 데이터를 미리 검토하고 계획하는 것이 포함됩니다. 또한 다양한 정보 소스에서 발견되는 정보 불일치를 검토하는 것도 포함됩니다. 시장 데이터는 시장 통계 및 일관된 모델을 사용하여 분석하고 추정합니다. 또한 시장 점유율 분석 및 주요 추세 분석은 시장 보고서의 주요 성공 요인입니다. 자세한 내용은 분석가에게 전화를 요청하거나 문의 사항을 드롭하세요.
DBMR 연구팀에서 사용하는 주요 연구 방법론은 데이터 마이닝, 시장에 대한 데이터 변수의 영향 분석 및 주요(산업 전문가) 검증을 포함하는 데이터 삼각 측량입니다. 데이터 모델에는 공급업체 포지셔닝 그리드, 시장 타임라인 분석, 시장 개요 및 가이드, 회사 포지셔닝 그리드, 특허 분석, 가격 분석, 회사 시장 점유율 분석, 측정 기준, 글로벌 대 지역 및 공급업체 점유율 분석이 포함됩니다. 연구 방법론에 대해 자세히 알아보려면 문의를 통해 업계 전문가에게 문의하세요.
사용자 정의 가능
Data Bridge Market Research는 고급 형성 연구 분야의 선두 주자입니다. 저희는 기존 및 신규 고객에게 목표에 맞는 데이터와 분석을 제공하는 데 자부심을 느낍니다. 보고서는 추가 국가에 대한 시장 이해(국가 목록 요청), 임상 시험 결과 데이터, 문헌 검토, 재생 시장 및 제품 기반 분석을 포함하도록 사용자 정의할 수 있습니다. 기술 기반 분석에서 시장 포트폴리오 전략에 이르기까지 타겟 경쟁업체의 시장 분석을 분석할 수 있습니다. 귀하가 원하는 형식과 데이터 스타일로 필요한 만큼 많은 경쟁자를 추가할 수 있습니다. 저희 분석가 팀은 또한 원시 엑셀 파일 피벗 테이블(팩트북)로 데이터를 제공하거나 보고서에서 사용 가능한 데이터 세트에서 프레젠테이션을 만드는 데 도움을 줄 수 있습니다.