North America In Situ Hybridization Devices Market
시장 규모 (USD 10억)
연평균 성장률 :
% 
 USD
0.52 Billion
USD
0.98 Billion
2024
2032
USD
0.52 Billion
USD
0.98 Billion
2024
2032
| 2025 –2032 | |
| USD 0.52 Billion | |
| USD 0.98 Billion | |
|
|
|
|
North America In-Situ Hybridization Devices Market Segmentation, By Technology (Fluorescence in-Situ Hybridization (FISH), Chromogenic in Situ Hybridization (CISH)), Probe Type (DNA, RNA), Device (Instruments, Consumables & Accessories, Software, Services), Application (Cancer, Cytogenetics, Developmental Biology, Infectious Diseases, Others), End User (Research & Diagnostic Laboratories, Pharmaceutical & Biotechnology Companies, Academic Institutes, CROs, Others), Country (U.S., Canada, Mexico) - Industry Trends and Forecast to 2032
North America In-Situ Hybridization Devices Market Size
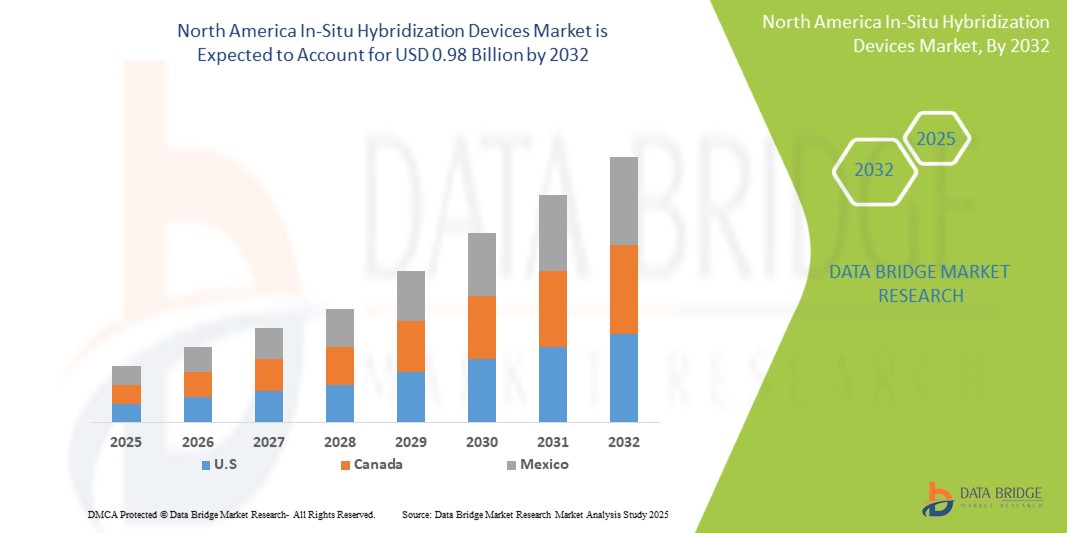
- The North America In-Situ Hybridization Devices Market was valued atUSD0.52 Billionin 2024and is expected to reachUSD0.98 Billionby 2032
- During the forecast period of 2025 to 2032 the market is likely to grow at aCAGR of 8.3%,primarily driven by the increasing prevalence of genetic disorders and cancers
- Key drivers of the North America In-Situ Hybridization Devices Market include the rising prevalence of genetic disorders and cancers, increasing adoption of personalized medicine, and advancements in diagnostic technologies.
North America In-Situ Hybridization Devices Market Analysis
- Market Growth and Trends: The North America in-situ hybridization devices market is experiencing significant growth driven by advancements in genetic research, increasing funding for biotechnology and genomics projects, and the expanding application of these devices in clinical diagnostics and research settings.
- Technological Advancements: Innovations in in-situ hybridization techniques, such as the development of more sensitive and efficient probes and the integration of automated systems, are enhancing the accuracy and efficiency of diagnostics, thereby boosting market adoption.
- For instance, The market is influenced by regulatory approvals from agencies like the FDA, which emphasize the need for safe and effective diagnostic tools. Compliance with stringent regulations and quality standards is crucial for manufacturers in this sector.
- Competitive Landscape: The North America in-situ hybridization devices market is characterized by a competitive landscape with key players focusing on partnerships, mergers, and acquisitions to enhance their product portfolios and expand market presence, while also investing in research and development to innovate new solutions.
Report Scope andNorth America In-Situ Hybridization Devices MarketSegmentation
|
Attributes |
North America In-Situ Hybridization Devices MarketKeyMarket Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include import export analysis, production capacity overview, production consumption analysis, price trend analysis, climate change scenario, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
North America In-Situ Hybridization Devices Market Trends
“Increasing Automation and Integration of Digital Technologies”
- Enhanced Efficiency and Accuracy: The trend towards automation in in-situ hybridization devices is significantly enhancing laboratory efficiency and accuracy. Automated systems reduce the potential for human error in sample processing and analysis, providing reproducible results. This is particularly important in high-throughput environments where consistent performance is critical for reliable diagnostics and research outcomes.
- Integration with Imaging Technologies: There is a growing trend to integrate in-situ hybridization devices with advanced imaging technologies, such as digital pathology and high-resolution microscopy. This integration allows for more detailed visualization and analysis of hybridization signals, enabling researchers to obtain deeper insights into cellular and molecular processes and facilitating more robust data interpretation.
- Development of User-Friendly Interfaces: The shift towards automation is also reflected in the creation of more user-friendly software interfaces that simplify the operation of in-situ hybridization devices. This trend aims to lower the barrier to entry for laboratories that are new to these technologies or have limited technical expertise, thereby broadening the market reach and fostering wider adoption across research institutions and diagnostic labs.
North America In-Situ Hybridization Devices MarketDynamics
Driver
“Rising Prevalence of Genetic and Infectious Diseases”
- Increasing Diagnostic Demand: The rising prevalence of genetic disorders and infectious diseases is driving the demand for accurate and efficient diagnostic tools. In-situ hybridization (ISH) devices are essential for detecting specific nucleic acid sequences in tissues, making them crucial for early diagnosis, which is vital in managing and treating these conditions effectively.
- Growing Cancer Research Initiatives: With cancer rates continuing to rise, there is an increasing focus on cancer research and the development of targeted therapies. In-situ hybridization devices play a significant role in cancer diagnostics by allowing pathologists to identify the presence of oncogenes or mutations directly in tumor samples. This helps in determining appropriate treatment plans and improving patient outcomes.
- Advancements in Molecular Pathology: The field of molecular pathology is advancing rapidly, with a greater emphasis on utilizing sophisticated techniques for disease characterization. In-situ hybridization devices are becoming more integral in research and clinical settings due to their ability to provide detailed spatial and contextual information about gene expression within tissue samples. This technological advancement, paired with the need for improved diagnostic tools, accelerates market growth.
Opportunity
“Expansion in Clinical and Research Applications”
- Rising Demand for Personalized Medicine: There is a growing emphasis on personalized medicine, which leverages genetic information to tailor treatments to individual patients. In-situ hybridization devices can play a critical role in identifying specific genetic markers and abnormalities, thus expanding their applications in cancer diagnostics, targeted therapies, and other personalized treatment approaches.
- Increased Funding for Genomic Research: Government and private investments in genomics and molecular biology are increasing, creating a conducive environment for the development and adoption of in-situ hybridization technologies. This funding can support innovative research projects, enhancing the demand for advanced in-situ hybridization devices and techniques in both academic and clinical settings.
- Adoption in Pathology and Diagnostics: As the diagnostic landscape evolves, there is an opportunity for in-situ hybridization devices to be increasingly utilized in pathology for detecting genetic alterations associated with diseases. The ability to provide spatial context to gene expression data makes these devices invaluable in understanding tumor microenvironments and disease progression, potentially leading to earlier and more accurate diagnoses.
Restraint/Challenge
“High Costs of In-Situ Hybridization Devices”
- Expensive Equipment and Maintenance: The initial investment required for in-situ hybridization devices can be quite high, which may deter smaller laboratories and institutions from adopting these technologies. Additionally, the ongoing costs associated with equipment maintenance, calibration, and servicing can further strain budgets, limiting accessibility for many potential users.
- Cost of Reagents and Consumables: In-situ hybridization procedures often require specialized reagents, probes, and consumables, which can be costly. These expenses can add up significantly over time, especially for laboratories that conduct large volumes of tests or research projects. The high cost of reagents can limit the frequency of testing and experimentation, impacting overall research productivity.
- Resource Allocation and Budget Constraints: Many research institutions and clinical laboratories face budget constraints that make it challenging to allocate sufficient funds for advanced technologies like in-situ hybridization devices. As healthcare budgets tighten and competition for research funding increases, labs may prioritize more established or less expensive methodologies, hindering the growth and adoption of in-situ hybridization technologies in the market.
North America In-Situ Hybridization Devices Market Scope
The market is segmented on the basis of technology, device, application, and end user.
|
Segmentation |
Sub-Segmentation |
|
By technology |
|
|
By device |
|
|
Byapplication |
|
|
By End User
|
|
North America In-Situ Hybridization Devices MarketRegional Analysis
“North America is the Dominant Region in theNorth America In-Situ Hybridization Devices Market”
- Advanced Research Infrastructure: North America, particularly the United States and Canada, is home to a robust research infrastructure that includes top-tier universities, research institutions, and healthcare facilities. This concentration of expertise and resources promotes innovation and the adoption of advanced techniques, such as in-situ hybridization, making the region a leader in the market.
- Strong Investment in Biotechnology and Life Sciences: The North American region benefits from significant investments in biotechnology, pharmaceuticals, and life sciences research. With the growing focus on personalized medicine, genomics, and molecular diagnostics, there is a heightened demand for in-situ hybridization devices, leading to increased market growth and development within the region.
- High Adoption Rate of Advanced Technologies: North America has a high adoption rate for cutting-edge healthcare technologies, supported by favorable regulatory environments and funding opportunities. This trend ensures that labs and institutions are more likely to integrate in-situ hybridization devices into their workflows, facilitating research and clinical applications. The presence of key market players and manufacturers further drives the availability of advanced in-situ hybridization technologies in the region.
- North America boasts advanced healthcare infrastructure, including comprehensive insurance systems and healthcare facilities. This infrastructure facilitates better access to Lancet and Pen Needles for patients, ensures effective reimbursement mechanisms, and supports broader market uptake of newly developed therapies for rare diseases.
“Asia-Pacific is Projected to Register the Highest Growth Rate in theNorth America In-Situ Hybridization Devices Market”
- Rising Investment in Biotechnology and Diagnostics: The Asia-Pacific region is experiencing a significant increase in investments in biotechnology, healthcare, and diagnostics sectors. Governments and private entities are funding research initiatives, infrastructure development, and the expansion of healthcare facilities. This influx of capital is expected to accelerate the adoption of advanced technologies, including in-situ hybridization devices, thereby driving market growth.
- Growing Research and Development Activities: Countries within the Asia-Pacific region are ramping up their research and development activities in molecular biology, genomics, and personalized medicine. The increasing focus on innovative research and the need for precise diagnostic tools are propelling the demand for in-situ hybridization devices. As more research institutions and academic centers adopt these technologies, the market is poised for rapid expansion.
- Rising Incidence of Genetic Disorders and Cancer: The Asia-Pacific region is witnessing an increase in the prevalence of genetic disorders and cancer, prompting a greater demand for accurate diagnostic tools and innovative research methodologies. In-situ hybridization is a crucial technique for studying gene expression and chromosomal abnormalities, making it essential in understanding and diagnosing such diseases. As healthcare providers and researchers seek better tools for diagnosis and treatment, the demand for in-situ hybridization devices is expected to soar, contributing to the region's high growth rate in the market.
North America In-Situ Hybridization Devices Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- Thermo Fisher Scientific Inc.
- Roche Diagnostics
- Agilent Technologies Inc.
- Bio-Techne Corporation
- Merck KGaA
- PerkinElmer Inc.
- F. Hoffmann-La Roche AG
- QIAGEN N.V.
- Abcam plc
- Illumina, Inc.
- BioVision Inc.
- Sigma-Aldrich (part of Merck Group)
- Bioneer Corporation
- VWR International, LLC (part of Avantor, Inc.)
- Exiqon (now part of Roche)
Latest Developments in North America In-Situ Hybridization Devices Market
- In October 2022, the commercialization of novel automated co-detection assays created particularly for the Roche DISCOVERY ULTRA Platform, allowing simultaneous identification of RNA and protein on the same tissue section, was announced by Bio-Techne Corporation as part of the further development of the Advanced Cell Diagnostics-branded RNAscope in ISH portfolio. Theradiag SA will provide autoimmune reagents and quality controls, while Quotient Limited is expected to grow its MosaiQ platform.
- in April 2021, Bio-Techne announced the commercial launch of its Novel DNAscope ISH assay for chromogenic detection of structural variations and DNA copy numbers. Unlike others, the commercially available assays, DNAScope enables high resolution and targeted detection of small genomic regions or single gene locus with its proprietary signal amplification system coupled with oligo probes.
SKU-
세계 최초의 시장 정보 클라우드 보고서에 온라인으로 접속하세요
- 대화형 데이터 분석 대시보드
- 높은 성장 잠재력 기회를 위한 회사 분석 대시보드
- 사용자 정의 및 질의를 위한 리서치 분석가 액세스
- 대화형 대시보드를 통한 경쟁자 분석
- 최신 뉴스, 업데이트 및 추세 분석
- 포괄적인 경쟁자 추적을 위한 벤치마크 분석의 힘 활용
연구 방법론
데이터 수집 및 기준 연도 분석은 대규모 샘플 크기의 데이터 수집 모듈을 사용하여 수행됩니다. 이 단계에는 다양한 소스와 전략을 통해 시장 정보 또는 관련 데이터를 얻는 것이 포함됩니다. 여기에는 과거에 수집한 모든 데이터를 미리 검토하고 계획하는 것이 포함됩니다. 또한 다양한 정보 소스에서 발견되는 정보 불일치를 검토하는 것도 포함됩니다. 시장 데이터는 시장 통계 및 일관된 모델을 사용하여 분석하고 추정합니다. 또한 시장 점유율 분석 및 주요 추세 분석은 시장 보고서의 주요 성공 요인입니다. 자세한 내용은 분석가에게 전화를 요청하거나 문의 사항을 드롭하세요.
DBMR 연구팀에서 사용하는 주요 연구 방법론은 데이터 마이닝, 시장에 대한 데이터 변수의 영향 분석 및 주요(산업 전문가) 검증을 포함하는 데이터 삼각 측량입니다. 데이터 모델에는 공급업체 포지셔닝 그리드, 시장 타임라인 분석, 시장 개요 및 가이드, 회사 포지셔닝 그리드, 특허 분석, 가격 분석, 회사 시장 점유율 분석, 측정 기준, 글로벌 대 지역 및 공급업체 점유율 분석이 포함됩니다. 연구 방법론에 대해 자세히 알아보려면 문의를 통해 업계 전문가에게 문의하세요.
사용자 정의 가능
Data Bridge Market Research는 고급 형성 연구 분야의 선두 주자입니다. 저희는 기존 및 신규 고객에게 목표에 맞는 데이터와 분석을 제공하는 데 자부심을 느낍니다. 보고서는 추가 국가에 대한 시장 이해(국가 목록 요청), 임상 시험 결과 데이터, 문헌 검토, 재생 시장 및 제품 기반 분석을 포함하도록 사용자 정의할 수 있습니다. 기술 기반 분석에서 시장 포트폴리오 전략에 이르기까지 타겟 경쟁업체의 시장 분석을 분석할 수 있습니다. 귀하가 원하는 형식과 데이터 스타일로 필요한 만큼 많은 경쟁자를 추가할 수 있습니다. 저희 분석가 팀은 또한 원시 엑셀 파일 피벗 테이블(팩트북)로 데이터를 제공하거나 보고서에서 사용 가능한 데이터 세트에서 프레젠테이션을 만드는 데 도움을 줄 수 있습니다.