North America Liver Fibrosis Treatment Market
시장 규모 (USD 10억)
연평균 성장률 :
% 
 USD
7.55 Billion
USD
16.87 Billion
2024
2032
USD
7.55 Billion
USD
16.87 Billion
2024
2032
| 2025 –2032 | |
| USD 7.55 Billion | |
| USD 16.87 Billion | |
|
|
|
북미 간 섬유증 치료 시장, 치료 유형(약물 및 수술/치료), 단계(F2, F1, F3, F4), 적응증(비알코올성 지방간염(NASH), B형 및 C형 간염 유발 섬유증, 알코올성 간 질환(ALD), 자가면역성 간 질환, 유전 질환 등), 성별(남성 및 여성), 최종 사용자(병원, 전문 클리닉, 클리닉, 외래 및 연구 센터 등), 유통 채널(직접 입찰 및 소매 판매) - 2032년까지의 산업 동향 및 전망
북미 간섬유증 치료 시장 규모
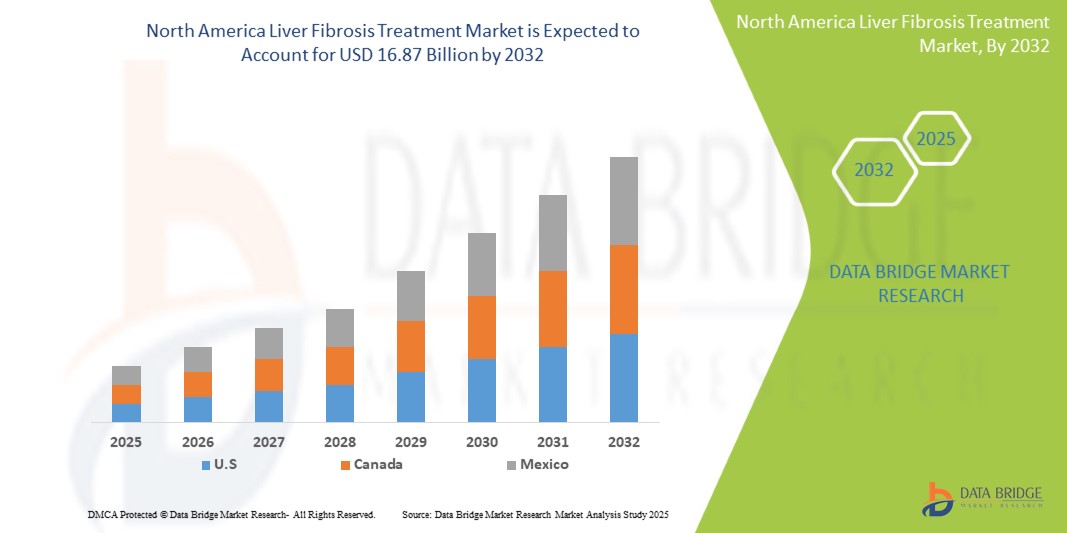
- 북미 간섬유증 치료 시장은 2024년에 75억 5천만 달러 규모로 평가되었으며 2032년에는 168억 7천만 달러 에 이를 것으로 예상됩니다 .
- 2025년부터 2032년까지의 예측 기간 동안 시장은 주로 자연 건강 솔루션에 대한 소비자 인식 증가에 따라 10.8%의 CAGR로 성장할 것으로 예상됩니다.
- 이러한 성장은 간 질환의 유병률 증가와 같은 요인에 의해 주도됩니다. 또한, 알코올 소비는 구매력 증가로 인해 증가합니다.
북미 간 섬유증 치료 시장 분석
- 간 질환의 증가로 인해 효과적인 치료 옵션이 필요한 환자 수가 늘어나고 있으며, 특히 B형 간염과 C형 간염과 같은 질환의 경우 더욱 그렇습니다. 알코올 소비 증가, 건강에 해로운 식습관, 비만율 증가와 같은 요인으로 인해 이러한 간 질환 진단을 받는 사람이 늘어나고 있습니다.
- 간 손상이 진행됨에 따라 간 섬유화 발생 위험이 증가하여 의료 시스템에 효과적인 치료 옵션을 제공해야 하는 상당한 부담이 가중됩니다. 이러한 수요 증가는 표적 치료제의 개발 및 공급을 촉진하여 의료 서비스 제공자들이 이 질환을 관리하기 위한 혁신적인 솔루션을 모색함에 따라 시장 성장을 촉진합니다.
- 더욱이 의료 종사자와 일반 대중의 인식 향상으로 인해 더 많은 사람들이 간 질환 초기 단계에서 검진을 받고 진단을 받고 있습니다. 비침습적 영상 검사 및 혈액 검사를 포함한 진단 기술의 발전으로 질병 진행 단계에서 간 섬유화를 조기에 발견하는 것이 가능해졌습니다. 이러한 조기 발견은 시기적절한 중재를 가능하게 하여 간 섬유화를 효과적으로 역전시키거나 관리하는 혁신적인 치료법에 대한 수요를 촉진합니다.
- 예를 들어, NCBI가 발표한 논문에 따르면 2023년 8월 기준, 간 질환으로 인한 사망자는 매년 200만 명에 달하며 전체 사망자의 4%(전 세계 사망자 25명 중 1명)를 차지합니다. 간 관련 사망의 약 3분의 2는 남성에게서 발생합니다. 이러한 놀라운 통계는 효과적인 치료 옵션의 시급성을 강조하고 간 질환으로 인한 심각한 공중 보건 부담을 부각시키며, 의료 시스템과 제약 회사들이 간 섬유증과 그 근본 원인을 특별히 표적으로 삼는 혁신적인 치료법 개발을 우선시하도록 유도합니다.
- 결과적으로 제약 회사와 의학 연구자들은 새로운 치료 옵션을 개발하는 데 투자할 수밖에 없으며, 이를 통해 이해관계자들이 간 질환과 관련 합병증의 발생률 증가에 대처하고자 노력함에 따라 시장이 활성화됩니다.
보고서 범위 및 북미 간 섬유증 치료 시장 세분화
|
속성 |
글로벌 간 섬유증 치료 시장 주요 시장 통찰력 |
|
다루는 세그먼트 |
|
|
포함 국가 |
|
|
주요 시장 참여자 |
|
|
시장 기회 |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include import export analysis, production capacity overview, production consumption analysis, price trend analysis, climate change scenario, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
North America Liver Fibrosis Treatment Market Trends
“Increasing Prevalence of Liver Diseases”
- The increasing prevalence of liver diseases is emerging as a significant North America health concern, contributing substantially to healthcare burdens worldwide
- Factors such as excessive alcohol consumption, rising obesity rates, viral hepatitis infections, and unhealthy lifestyles are leading to a surge in conditions like fatty liver disease, cirrhosis, and liver cancer
- The growing incidence of non-alcoholic fatty liver disease (NAFLD), particularly linked to diabetes and metabolic syndrome, is also alarming. As a result, there is an increased demand for early diagnosis, effective treatment options, and public health initiatives. This trend is expected to drive innovation and growth in the liver disease treatment market
North America Liver Fibrosis Treatment Market Dynamics
Drivers
“Rising Consumption of Alcohol”
- Rising alcohol consumption is a significant contributor to the North America liver fibrosis treatment market due to its direct correlation with the incidence of liver diseases, particularly Alcoholic Liver Disease (ALD)
- As more individuals consume alcohol regularly and in larger quantities, the risk of developing liver-related complications, including liver fibrosis and cirrhosis, increases significantly
- Chronic alcoholism leads to inflammation, fat accumulation, and, ultimately, fibrosis as the liver undergoes repeated damage and repair
- This growing prevalence of alcohol-related liver conditions creates a pressing demand for effective treatments and management strategies to help mitigate liver damage and improve patient outcomes
For instance,
- In October 2024, as per an article published by the International Journal of Mental Health Systems, the prevalence of alcohol consumption was 54.5% and 47.7% at the baseline and follow-up, respectively. Moreover, 12% of men reported to have newly started drinking. This prevalence of alcohol consumption leads to an increased incidence of liver fibrosis North Americaly, potentially impacting market growth
- In June 2024, as per STAT, alcohol-related deaths are on the rise, and experts are particularly concerned about an increase among young people and women. The U.S. saw a 25.5% spike in alcohol-related deaths between 2019 and 2020 — accounting for 3% of all deaths. Moreover, the largest increases in alcohol-related deaths were among people 25 to 34 and 35 to 44 years old, wherein deaths in both groups increased by over 37%
- Moreover, the increasing societal acceptance and normalization of alcohol consumption, particularly in younger demographics, further exacerbate the issue, leading to a greater number of individuals at risk for liver fibrotic changes
- This trend promotes the growth of the liver fibrosis treatment market and emphasizes the importance of public health initiatives aimed at reducing alcohol consumption and preventing liver disease
Opportunities
“Emerging Technologies and Advanced Treatments in Liver Fibrosis Management”
-
Emerging technologies like gene therapy, targeted molecular therapies, and biologic agents are transforming the treatment landscape
-
Recent innovations focus on drugs that specifically target fibrosis progression pathways, such as FXR agonists, TGF-β inhibitors, and anti-inflammatory agents. In addition, non-invasive diagnostic tools like elastography are improving early detection
-
These advancements offer more effective, personalized treatments for conditions such as NASH (Non-Alcoholic Steatohepatitis) and cirrhosis, leading to better patient outcomes
-
In February 2024, an article published in Springer Nature, The article reviews emerging approaches for diagnosing and inhibiting liver fibrogenesis. Advances include non-invasive biomarkers, imaging technologies, and cell therapies like mesenchymal stem cells. Promising antifibrotic drugs, including pirfenidone and obeticholic acid, along with innovations in tissue engineering, nanotechnology, and microfluidic models, show potential for personalized, precision treatments
-
A September 2021, article by NCBI highlighted that Emerging technologies in liver fibrosis treatment focus on advanced therapies targeting molecular pathways like hepatic stellate cell activation. Innovations such as gene therapy, biologics, small molecule inhibitors, and non-invasive diagnostics improve early detection and treatment. Stem cell therapies and tissue engineering also offer promise for reversing fibrosis and enhancing recovery
-
With the continuous evolution of treatment strategies and diagnostic technologies, liver fibrosis therapies are advancing rapidly
-
These innovations provide hope for better management of liver diseases, ensuring that patients have access to more effective, personalized treatments with fewer side effects, ultimately improving North America health outcomes
Restraints/Challenge
“Limited Awareness of Liver Diseases”
- 간 질환에 대한 인식 부족은 조기 진단과 적절한 개입을 어렵게 만듭니다. 많은 사람들이 간 질환과 관련된 위험 요인과 증상을 인지하지 못하고, 모호한 질병 징후를 덜 심각한 다른 질환으로 돌리는 경우가 많습니다.
- 이러한 지식 부족으로 인해 질병이 간 섬유증이나 간경변과 같이 진행되어 치료 옵션이 더 복잡해지고 효과가 떨어질 때까지 의료 상담이 지연됩니다.
- 결과적으로 후기 진단은 성공적인 치료 결과의 가능성을 감소시키고 시기적절한 치료를 추구하는 환자 집단을 제한함으로써 전체 시장 성장을 제한합니다.
예를 들어,
- 2024년 4월, 루핀 박사는 환자들이 질병이 심각한 단계에 도달할 때까지 자신의 상태를 인지하지 못한다고 밝혔으며, 이는 간 건강 인식, 진단 및 관리에 대한 접근 방식을 재평가해야 할 시급한 필요성을 촉발했습니다. 그러나 간 건강 관리의 중요한 장애물은 간 질환과 관련 위험 요인에 대한 이해가 부족하다는 것입니다.
- 2021년 7월, 5개의 전국 건강 및 영양 조사(National Health and Nutrition Examination Survey)에 참여한 18세 이상 성인 11,700명을 대상으로 한 연구에 따르면, 미국 성인 NAFLD 환자의 약 96%가 간 질환을 앓고 있다는 사실을 인지하지 못하고 있었으며, 특히 젊은 성인의 경우 더욱 그러했습니다. 따라서 간 건강에 대한 인식과 교육을 강화하는 것은 환자 치료 결과를 개선하고 간 섬유화 관리 및 치료 시장 확대에 대한 보다 적극적인 접근 방식을 촉진하는 데 매우 중요합니다.
- 2021년 1월, Springer Nature는 '제2형 당뇨병 환자의 간 손상 인식 부족'이라는 연구 논문에서 수행된 연구 분석에 포함된 825명의 환자 중 지방간 환자의 8.1%(95% 신뢰 구간 5.1%~12.7%)가 간 질환을 인지하고 있었다고 보고했습니다. 더욱이, 제2형 당뇨병을 앓고 있는 미국 성인을 대상으로 한 전국 대표 표본에서 진행성 간 섬유증의 유병률은 높습니다. 진행성 섬유증 환자 중 간 질환을 인지하는 사람은 20% 미만입니다.
- 제한된 인식은 환자의 잠재적 치료 결과를 방해하고 조기에 의료 서비스를 이용하는 개인 수를 줄임으로써 시장 성장을 저해합니다.
- 따라서 간 건강에 대한 인식과 교육을 높이는 것은 조기 진단을 개선하고 치료 효능을 강화하며 궁극적으로 간 섬유증 치료에 대한 보다 강력한 시장을 육성하는 데 매우 중요합니다.
북미 간 섬유증 치료 시장 범위
시장은 제품 유형, 치료, 출처 기준, 적용, 투여 경로, 구매 방식, 연령대, 성별, 최종 사용자 및 유통 채널을 기준으로 세분화됩니다.
|
분할 |
하위 세분화 |
|
치료 유형별 |
|
|
단계별 로 |
|
|
표시 에 의해 |
|
|
최종 사용자 별 |
|
|
유통 채널 별 |
|
북미 간 섬유증 치료 시장 지역 분석
“미국은 간섬유증 치료 시장의 주요 국가입니다.”
- 미국은 강력한 의료 인프라, 간 관련 질환의 높은 유병률, 조기 진단 및 개입 전략에 대한 강력한 집중으로 북미 간 섬유증 치료 시장을 선도하고 있습니다.
- 이 나라는 광범위한 의료 보장, 확립된 보상 정책, 비침습적 진단 및 치료 옵션에 대한 수요 증가로 혜택을 받고 있습니다.
- 임상 연구 분야에서 미국이 보여준 리더십과 간 질환 인식 캠페인에 대한 적극적인 참여는 시장 성장을 더욱 뒷받침합니다.
- 첨단 치료법의 채택 증가, 환자 인식 제고, 만성 간 질환을 해결하기 위한 정부 이니셔티브는 미국이 이 지역에서 우위를 점하는 데 기여합니다.
“미국, 가장 높은 성장률 기록할 듯”
- 미국은 또한 섬유증 바이오마커의 지속적인 혁신, 제약 회사의 R&D 투자 증가, 알코올 관련 및 비알코올성 지방간 질환의 증가율 증가에 힘입어 가장 빠르게 성장하는 시장입니다.
- 이러한 요소들은 미국을 북미 간 섬유증 치료의 중심 허브로 자리매김하게 했으며, 이를 통해 미국은 이 지역에서 가장 크고 가장 빠르게 확장되는 시장이 되었습니다.
북미 간섬유증 치료 시장 점유율
시장 경쟁 구도는 경쟁사별 세부 정보를 제공합니다. 여기에는 회사 개요, 회사 재무 상태, 매출 창출, 시장 잠재력, 연구 개발 투자, 신규 시장 진출, 북미 지역 사업 현황, 생산 시설 및 설비, 생산 능력, 회사의 강점과 약점, 제품 출시, 제품 종류 및 범위, 응용 분야별 우위 등이 포함됩니다. 위에 제공된 데이터는 해당 회사의 시장 집중도와 관련된 내용만 포함합니다.
시장에서 활동하는 주요 시장 리더는 다음과 같습니다.
- F. 호프만-라 로슈 유한회사(스위스)
- 애벗(미국)
- 라 레논 헬스케어 주식회사 유한회사(인도)
- GENFIT SA(프랑스)
- 마드리갈 제약(미국)
- 알리고스 테라퓨틱스(미국)
- 화이자(미국)
- Enanta Pharmaceuticals, Inc.(미국)
- 브리스톨-마이어스 스퀴브 회사(미국)
- Vertex Pharmaceuticals Incorporated(미국)
- 다케다제약 주식회사(일본)
- 헤피온제약(미국)
- 에코센스(프랑스)
- Galectin Therapeutics, Inc. (미국)
- 코나투스 파마슈티컬스(미국)
- 트바르디 테라퓨틱스(미국)
- 바이킹 테라퓨틱스(미국)
- Calliditas Therapeutics AB (스웨덴)
- 노보메딕스(미국)
- 갈렉토 바이오텍(덴마크)
- 필란트 테라퓨틱스(Pilant Therapeutics, Inc.) (미국)
- 사기메트 바이오사이언스(미국)
- Gyre Therapeutics, Inc. (미국)
- Akero Therapeutics, Inc. (미국)
- CureVac SE(독일)
- Novo Nordisk A/S(덴마크)
- Ipsen Pharma(프랑스)
- AdAlta Limited(호주)
- 알렌티스 테라퓨틱스 AG(스위스)
- 길리어드 사이언스 주식회사(미국)
- AbbVie Inc.(미국)
- 머크앤코(미국)
- 노바티스 AG(스위스)
- Intercept Pharmaceuticals, Inc. (미국)
북미 간 섬유증 치료 시장의 최신 동향
- 2024년 6월, 길리어드 사이언스는 밀라노에서 개최된 2024 유럽 간학회(EASL) 총회에서 원발성 담즙성 담관염(PBC), B형 간염(HBV), 델타 간염 바이러스(HDV) 등 간 질환에 초점을 맞춘 새로운 연구를 발표했습니다. 주요 발표 내용으로는 PBC에 대한 셀라델파(seladelpar)의 ASSURE 연구 장기 데이터, HBV 간암 예방을 위한 테노포비르(tenofovir)의 결과, 그리고 HDV에 대한 헵클루덱스(Hepcludex)의 MYR204 및 MYR301 연구 결과가 포함됩니다. 이 연구는 간 질환 치료 옵션 발전에 대한 길리어드의 헌신을 강조합니다.
- 2024년 10월, Intercept Pharmaceuticals, Inc.는 원발성 담즙성 담관염(PBC) 치료에서 인종적 차이와 불균형을 탐구하기 위한 지속적인 노력에 대해 논의했습니다. Intercept는 다양한 인구 집단이 PBC의 진단, 치료 및 관리에 있어 서로 다른 어려움에 직면할 수 있다는 점을 다루고 있습니다. Intercept는 이러한 불균형을 탐구함으로써 모든 환자의 치료 접근성과 치료 결과를 개선하고, 섬유증을 포함한 간 질환의 효과적인 치료에 대한 장벽을 낮추는 것을 목표로 합니다.
- 2022년 11월, 미국 식품의약국(FDA)은 12세 이상 대상성 간 질환 소아 환자의 만성 B형 간염 바이러스(HBV) 감염 치료에 베믈리디(Vemlidy, 테노포비르 알라페나미드)를 승인했습니다. 이번 승인은 2016년 성인 만성 B형 간염 환자를 대상으로 처음 승인된 베믈리디의 사용 기간을 연장하는 것입니다. 이번 승인은 이 젊은 환자군에서 베믈리디의 효능과 안전성을 입증하는 2상 임상시험 결과를 기반으로 합니다.
- 2022년 9월, 길리어드 사이언스는 면역 억제 수용체를 표적으로 하는 작용제를 통해 면역 균형을 회복하는 데 주력하는 영국 생명공학 기업 미로바이오(MiroBio)를 인수했습니다. 약 4억 5백만 달러 규모의 이번 인수를 통해 길리어드는 미로바이오의 발굴 플랫폼과 면역 억제 수용체 작용제 포트폴리오를 확보하게 되었습니다. 미로바이오의 주요 연구 항체인 MB272는 면역 세포를 표적으로 삼아 염증성 면역 반응을 억제하며 현재 임상 1상 시험 중입니다. 이번 인수는 만성 면역 매개 질환 치료에 대한 길리어드의 노력을 강화할 것입니다.
- 2021년 3월, 길리어드 사이언스와 노보 노디스크는 비알코올성 지방간염(NASH) 치료 분야에서 협력을 확대하며 2b상 임상시험을 시작했습니다. 이 연구는 노보 노디스크의 GLP-1 수용체 작용제인 세마글루타이드와 길리어드의 실로펙서(FXR 작용제) 및 피르소코스타트(ACC 억제제) 병용 투여 시 NASH로 인한 간경변 환자에서 안전성과 유효성을 평가합니다. 이 임상시험은 간 섬유화 및 NASH 해소에 대한 두 약물의 영향을 평가할 예정이며, 2021년 하반기부터 환자 모집이 시작될 예정입니다.
SKU-
세계 최초의 시장 정보 클라우드 보고서에 온라인으로 접속하세요
- 대화형 데이터 분석 대시보드
- 높은 성장 잠재력 기회를 위한 회사 분석 대시보드
- 사용자 정의 및 질의를 위한 리서치 분석가 액세스
- 대화형 대시보드를 통한 경쟁자 분석
- 최신 뉴스, 업데이트 및 추세 분석
- 포괄적인 경쟁자 추적을 위한 벤치마크 분석의 힘 활용
목차
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF THE NORTH AMERICA LIVER FIBROSIS TREATMENT MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATIONS
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 DBMR TRIPOD DATA VALIDATION MODEL
2.5 MULTIVARIATE MODELLING
2.6 TREATMENT TYPE LIFELINE CURVE
2.7 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.8 DBMR MARKET POSITION GRID
2.9 MARKET END USER COVERAGE GRID
2.1 VENDOR SHARE ANALYSIS
2.11 SECONDARY SOURCES
2.12 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTAL ANALYSIS
4.2 PORTERS FIVE FORCES ANALYSIS
4.2.1 LIVER TRANSPLANTATION VOLUME AND THEIR COST FOR LIVER FIBROSIS BY COUNTRY
4.2.2 ENDOSCOPIC & MINIMALLY INVASIVE PROCEDURES FOR LIVER FIBROSIS: VOLUME AND COST BY COUNTRY
4.2.3 PARTIAL HEPATECTOMY (LIVER RESECTION) COST BY COUNTRY
4.2.4 CELL-BASED THERAPY COST FOR LIVER FIBROSIS TREATMENT BY COUNTRY
4.3 EPIDEMIOLOGY
4.3.1 INCIDENCE OF ALL BY GENDER
4.3.2 TREATMENT RATE
4.3.3 TREATMENT RATE
4.3.4 DRUG ADHERENCE AND THERAPY SWITCH MODEL
4.3.5 PATIENT TREATMENT SUCCESS RATES
4.4 MARKETED DRUG ANALYSIS
4.5 DEMOGRAPHIC TRENDS: IMPACTS ON ALL INCIDENCE RATES
4.5.1 PATIENT FLOW DIAGRAM
4.5.2 KEY PRICING STRATEGIES
4.5.3 KEY PATIENT ENROLLMENT STRATEGIES
5 NORTH AMERICA LIVER FIBROSIS TREATMENT MARKET: REGULATIONS
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 INCREASING PREVALENCE OF LIVER DISEASES
6.1.2 RISING CONSUMPTION OF ALCOHOL
6.1.3 RISING LIVER TRANSPLANTATION RATES
6.1.4 GROWING INCIDENCE OF NON-ALCOHOLIC FATTY LIVER DISEASE (NAFLD) & NASH
6.2 RESTRAINTS
6.2.1 LIMITED AWARENESS OF LIVER DISEASES
6.2.2 REGULATORY CHALLENGES
6.3 OPPORTUNITIES
6.3.1 EMERGING TECHNOLOGIES AND ADVANCED TREATMENTS IN LIVER FIBROSIS MANAGEMENT
6.3.2 PROGRESS IN PIPELINE PRODUCTS FOR LIVER FIBROSIS TREATMENT
6.3.3 STRATEGIC MERGERS AND ACQUISITIONS AMONG THE KEY PLAYERS
6.4 CHALLENGES
6.4.1 LACK OF EFFECTIVE AND APPROVED ANTI-FIBROTIC DRUGS
6.4.2 HIGH COST OF TREATMENTS IN LIVER FIBROSIS CARE
7 NORTH AMERICA LIVER FIBROSIS TREATMENT MARKET, BY TREATMENT TYPE
7.1 OVERVIEW
7.2 MEDICATION
7.2.1 ANTIVIRAL AGENTS
7.2.1.1 VELPATASVIR/SOFOSBUVIR
7.2.1.2 TENOFOVIR
7.2.1.3 LEDIPASVIR/SOFOSBUVIR
7.2.1.4 SOFOSBUVIR
7.2.1.5 ENTECAVIR
7.2.2 ANTIFIBROTIC AGENTS
7.2.2.1 OBETICHOLIC ACID
7.2.2.2 TGF-Β INHIBITORS
7.2.2.3 CONNECTIVE TISSUE GROWTH FACTOR (CTGF) INHIBITORS
7.2.2.4 LYSYL OXIDASE-LIKE 2 (LOXL2) INHIBITORS
7.2.2.5 OTHERS
7.2.3 ANTI-INFLAMMATORY DRUGS
7.2.3.1 CORTICOSTEROIDS
7.2.3.1.1 PREDNISONE
7.2.3.1.2 DEXAMETHASONE
7.2.3.2 TUMOR NECROSIS FACTOR (TNF) INHIBITORS
7.2.3.2.1 INFLIXIMAB
7.2.3.2.2 ETANERCEPT
7.2.3.3 INTERLEUKIN (IL) INHIBITORS
7.2.3.3.1 IL-6 INHIBITORS (TOCILIZUMAB)
7.2.3.3.2 IL-1 INHIBITORS (ANAKINRA)
7.2.4 IMMUNOSUPPRESSANTS
7.2.4.1 MYCOPHENOLATE MOFETIL
7.2.4.2 TACROLIMUS
7.2.4.3 CYCLOSPORINE
7.2.5 MARKETED DRUGS
7.2.5.1 VELPATASVIR/SOFOSBUVIR
7.2.5.2 TENOFOVIR
7.2.5.3 LEDIPASVIR/SOFOSBUVIR
7.2.5.4 OBETICHOLIC ACID (OCA)
7.2.5.5 SOFOSBUVIR
7.2.5.6 PIRFENIDONE
7.2.5.7 OTHERS
7.2.6 PIPELINE DRUGS
7.2.7 BRANDED DRUGS
7.2.7.1 EPCLUSA
7.2.7.2 VIREAD AND VEMLIDY
7.2.7.3 OCALIVA
7.2.7.4 HARVONI
7.2.7.5 SOVALDI
7.2.7.6 BARACLUDE
7.2.7.7 ACTOS
7.2.7.8 OTHERS
7.2.8 GENERIC DRUGS
7.2.9 ORAL
7.2.10 PARENTERAL
7.2.11 OTHERS
7.3 SURGERY/THERAPY
7.3.1 LIVER TRANSPLANTATION
7.3.2 ORTHOTOPIC LIVER TRANSPLANT (OLT)
7.3.3 LIVING DONOR LIVER TRANSPLANT (LDLT)
7.3.4 SPLIT LIVER TRANSPLANTATION
7.3.5 DOMINO LIVER TRANSPLANT
7.3.6 ENDOSCOPIC & MINIMALLY INVASIVE PROCEDURES
7.3.6.1 ENDOSCOPIC VARICEAL LIGATION (EVL)
7.3.6.2 TRANSJUGULAR INTRAHEPATIC PORTOSYSTEMIC SHUNT (TIPS)
7.3.6.3 LIVER ABLATION PROCEDURES
7.3.6.3.1 RADIOFREQUENCY ABLATION (RFA)
7.3.6.3.2 MICROWAVE ABLATION (MWA)
7.3.7 PARTIAL HEPATECTOMY (LIVER RESECTION)
7.3.7.1 SEGMENTAL RESECTION
7.3.7.2 LOBECTOMY
7.3.7.3 WEDGE RESECTION
7.3.8 CELL-BASED THERAPY
7.3.8.1 STEM CELL THERAPY
7.3.8.1.1 MESENCHYMAL STEM CELLS (MSCS)
7.3.8.1.2 HEMATOPOIETIC STEM CELLS (HSCS)
7.3.8.2 GENE THERAPY
7.3.8.2.1 CRISPR-BASED LIVER REGENERATION
7.3.8.2.2 HEPATIC STELLATE CELL (HSC) INHIBITORS
7.3.8.2.3 SIRNA-BASED THERAPIES
7.3.8.2.4 HEPATOCYTE APOPTOSIS INHIBITORS
7.3.8.2.4.1 OXIDATIVE STRESS INHIBITORS
7.3.8.2.4.2 EMRICASAN
7.3.8.2.4.3 PENTOXIFYLLINE
7.3.8.2.4.4 LOSARTAN
7.3.8.2.4.5 METHYL FERULIC ACID
7.3.8.2.4.6 OTHERS
7.4 OTHERS
8 NORTH AMERICA LIVER FIBROSIS TREATMENT MARKET, BY STAGES
8.1 OVERVIEW
8.2 F2
8.3 F1
8.4 F3
8.5 F4
9 NORTH AMERICA LIVER FIBROSIS TREATMENT MARKET, BY INDICATION
9.1 OVERVIEW
9.2 NON-ALCOHOLIC STEATOHEPATITIS (NASH)
9.3 HEPATITIS B & C-INDUCED FIBROSIS
9.3.1 CHRONIC HEPATITIS B VIRUS (HBV) FIBROSIS
9.3.2 CHRONIC HEPATITIS C VIRUS (HCV) FIBROSIS
9.4 ALCOHOLIC LIVER DISEASE (ALD)
9.5 AUTOIMMUNE LIVER DISEASES
9.5.1 AUTOIMMUNE HEPATITIS (AIH)
9.5.2 PRIMARY BILIARY CHOLANGITIS (PBC)
9.5.3 PRIMARY SCLEROSING CHOLANGITIS (PSC)
9.6 GENETIC DISORDERS
9.6.1 HEMOCHROMATOSIS
9.6.2 WILSON’S DISEASE
9.6.3 ALPHA-1 ANTITRYPSIN DEFICIENCY
9.7 OTHERS
10 NORTH AMERICA LIVER FIBROSIS TREATMENT MARKET, BY GENDER
10.1 OVERVIEW
10.2 MALE
10.2.1 40-55 YEARS
10.2.2 ABOVE 55 YEARS
10.2.3 BELOW 40 YEARS
10.3 FEMALE
10.3.1 ABOVE 55 YEARS
10.3.2 40-55 YEARS
10.3.3 BELOW 40 YEARS
11 NORTH AMERICA LIVER FIBROSIS TREATMENT MARKET, BY END USER
11.1 OVERVIEW
11.2 HOSPITALS
11.2.1 PUBLIC HOSPITALS
11.2.2 PRIVATE HOSPITALS
11.3 SPECIALTY CLINICS
11.3.1 HEPATOLOGY CLINICS
11.3.2 GASTROENTEROLOGY CLINICS
11.4 CLINICS
11.5 AMBULATORY AND RESEARCH CENTERS
11.6 OTHERS
12 NORTH AMERICA LIVER FIBROSIS TREATMENT MARKET, BY DISTRIBUTION CHANNEL
12.1 OVERVIEW
12.2 DIRECT TENDER
12.2.1 RETAIL SALES
12.2.1.1 HOSPITAL PHARMACY
12.2.1.2 RETAIL PHARMACY
12.2.1.3 ONLINE PHARMACY
13 NORTH AMERICA LIVER FIBROSIS TREATMENT MARKET, BY REGION
13.1 NORTH AMERICA
13.1.1 U.S.
13.1.2 CANADA
13.1.3 MEXICO
14 NORTH AMERICA LIVER FIBROSIS TREATMENT MARKET: COMPANY LANDSCAPE
14.1 COMPANY SHARE ANALYSIS: GLOBAL
15 SWOT ANALYSIS
16 COMPANY PROFILES
16.1 GILEAD SCIENCES, INC.
16.1.1 COMPANY SNAPSHOT
16.1.2 REVENUE ANALYSIS
16.1.3 COMPANY SHARE ANALYSIS
16.1.4 PRODUCT PORTFOLIO
16.1.5 RECENT DEVELOPMENT/NEWS
16.2 ABBVIE, INC.
16.2.1 COMPANY SNAPSHOT
16.2.2 REVENUE ANALYSIS
16.2.3 COMPANY SHARE ANALYSIS
16.2.4 PRODUCT PORTFOLIO
16.2.5 RECENT DEVELOPMENT
16.3 MERCK & CO, INC.
16.3.1 COMPANY SNAPSHOT
16.3.2 REVENUE ANALYSIS
16.3.3 COMPANY SHARE ANALYSIS
16.3.4 PRODUCT PORTFOLIO
16.3.5 RECENT DEVELOPMENT
16.4 NOVARTIS AG
16.4.1 COMPANY SNAPSHOTS
16.4.2 REVENUE ANALYSIS AND SEGMENTED ANALYSIS
16.4.3 COMPANY SHARE ANALYSIS
16.4.4 PRODUCT PORTFOLIO
16.4.5 PIPELINE PRODUCT PORTFOLIO
16.4.6 RECENT DEVELOPMENT
16.5 INTERCEPT PHARMACEUTICALS, INC.
16.5.1 COMPANY SNAPSHOTS
16.5.2 COMPANY SHARE ANALYSIS
16.5.3 REVENUE ANALYSIS AND SEGMENTED ANALYSIS
16.5.4 PRODUCT PORTFOLIO
16.5.5 PIPELINE PRODUCT PORTFOLIO
16.5.6 RECENT NEWS
16.6 ABBOTT
16.6.1 COMPANY SNAPSHOT
16.6.2 REVENUE ANALYSIS
16.6.3 PRODUCT PORTFOLIO
16.6.4 1.1.5 RECENT DEVELOPMENT
16.7 ALIGOS THERAPEUTICS
16.7.1 COMPANY SNAPSHOT
16.7.2 REVENUE ANALYSIS
16.7.3 PRODUCT PORTFOLIO
16.7.4 RECENT DEVELOPMENT
16.8 ALNICHE LIFE SCIENCES PVT. LTD.
16.8.1 COMPANY SNAPSHOT
16.8.2 PRODUCT PORTFOLIO
16.8.3 RECENT DEVELOPMENT
16.9 ALENTIS THERAPEUTICS AG
16.9.1 COMPANY SNAPSHOT
16.9.2 PIPELINE PRODUCT PORTFOLIO
16.9.3 RECENT DEVELOPMENT
16.1 ADALTA LIMITED
16.10.1 COMPANY SNAPSHOT
16.10.2 REVENUE ANALYSIS
16.10.3 PIPELINE PRODUCT PORTFOLIO
16.10.4 RECENT NEWS
16.11 AKERO THERAPEUTICS, INC.
16.11.1 COMPANY SNAPSHOT
16.11.2 REVENUE ANALYSIS
16.11.3 PIPELINE PRODUCT PORTFOLIO
16.11.4 RECENT DEVELOPMENT
16.12 BRISTOL-MYERS SQUIBB
16.12.1 COMPANY SNAPSHOT
16.12.2 REVENUE ANALYSIS
16.12.3 PRODUCT PORTFOLIO
16.12.4 RECENT DEVELOPMENT
16.13 CALLIDITAS THERAPEUTICS AB
16.13.1 COMPANY SNAPSHOT
16.13.2 PIPELINE PRODUCT PORTFOLIO
16.13.3 RECENT DEVELOPMENT
16.14 CUREVAC SE
16.14.1 COMPANY SNAPSHOT
16.14.2 REVENUE ANALYSIS
16.14.3 PRODUCT PORTFOLIO
16.14.4 RECENT DEVELOPMENT
16.15 CONATUSPHARMA
16.15.1 COMPANY SNAPSHOT
16.15.2 PRODUCT PORTFOLIO
16.15.3 RECENT DEVELOPMENT/NEWS
16.16 ENANTA PHARMACEUTICALS, INC.
16.16.1 COMPANY SNAPSHOT
16.16.2 REVENUE ANALYSIS
16.16.3 PRODUCT PORTFOLIO
16.16.4 RECENT DEVELOPMENT
16.17 ECHOSENS
16.17.1 COMPANY SNAPSHOT
16.17.2 PRODUCT PORTFOLIO
16.17.3 RECENT DEVELOPMENT/NEWS
16.18 F. HOFFMANN-LA ROCHE LTD
16.18.1 COMPANY SNAPSHOT
16.18.2 REVENUE ANALYSIS
16.18.3 COMPANY SHARE ANALYSIS
16.18.4 PRODUCT PORTFOLIO
16.18.5 RECENT DEVELOPMENT
16.19 GALECTO BIOTECH
16.19.1 COMPANY SNAPSHOT
16.19.2 REVENUE ANALYSIS
16.19.3 PIPELINE PRODUCT PORTFOLIO
16.19.4 RECENT DEVELOPMENT/NEWS
16.2 GALECTIN THERAPEUTICS, INC.
16.20.1 COMPANY SNAPSHOTS
16.20.2 REVENUE ANALYSIS AND SEGMENTAL ANALYSIS
16.20.3 PIPELINE PRODUCT PORTFOLIO
16.20.4 RECENT DEVELOPMENT
16.21 GYRE THERAPEUTICS, INC.
16.21.1 COMPANY SNAPSHOT
16.21.2 REVENUE ANALYSIS AND SEGMENTED ANALYSIS
16.21.3 PRODUCT PORTFOLIO
16.21.4 RECENT DEVELOPMENT/NEWS
16.22 GENFIT SA
16.22.1 COMPANY SNAPSHOTS
16.22.2 REVENUE ANALYSIS AND SEGMENTED ANALYSIS
16.22.3 PIPELINE PRODUCT PORTFOLIO
16.22.4 RECENT DEVELOPMENT
16.23 HEPION PHARMACEUTICALS
16.23.1 COMPANY SNAPSHOT
16.23.2 REVENUE ANALYSIS
16.23.3 PIPELINE PORTFOLIO
16.23.4 RECENT DEVELOPMENT
16.24 IPSEN PHARMA
16.24.1 COMPANY SNAPSHOT
16.24.2 REVENUE ANALYSIS
16.24.3 PIPELINE PRODUCT PORTFOLIO
16.24.4 RECENT NEWS/DEVELOPMENTS
16.25 LA RENON HEALTHCARE PVT. LTD.
16.25.1 COMPANY SNAPSHOT
16.25.2 PRODUCT PORTFOLIO
16.25.3 RECENT DEVELOPMENT
16.26 MADRIGAL PHARMACEUTICALS
16.26.1 COMPANY SNAPSHOTS
16.26.2 REVENUE ANALYSIS AND SEGMENTED ANALYSIS
16.26.3 PRODUCT PORTFOLIO
16.26.4 RECENT DEVELOPMENT
16.27 NOVO NORDISK A/S
16.27.1 COMPANY SNAPSHOT
16.27.2 REVENUE ANALYSIS
16.27.3 PIPELINE PRODUCT PORTFOLIO
16.27.4 RECENT DEVELOPMENT
16.28 NOVOMEDIX
16.28.1 COMPANY SNAPSHOT
16.28.2 PIPELINE PRODUCT PORTFOLIO
16.28.3 RECENT DEVELOPMENT
16.29 PILANT THERAPEUTICS, INC.
16.29.1 COMPANY SNAPSHOT
16.29.2 REVENUE ANALYSIS
16.29.3 PIPELINE PRODUCT PORTFOLIO
16.29.4 RECENT NEWS
16.3 PFIZER INC.
16.30.1 COMPANY SNAPSHOT
16.30.2 REVENUE ANALYSIS
16.30.3 PIPELINE PRODUCT PORTFOLIO
16.30.4 RECENT DEVELOPMENT/NEWS
16.31 SAGIMET BIOSCIENCES
16.31.1 COMPANY SNAPSHOTS
16.31.2 REVENUE ANALYSIS
16.31.3 1.1.4 PRODUCT PORTFOLIO
16.31.4 RECENT DEVELOPMENT/NEWS
16.32 TAKEDA PHARMACEUTICAL COMPANY LIMITED
16.32.1 COMPANY SNAPSHOT
16.32.2 REVENUE ANALYSIS
16.32.3 PIPELINE PRODUCT PORTFOLIO
16.32.4 PRODUCT PORTFOLIO
16.32.5 RECENT DEVELOPMENT
16.33 TVARDI THERAPEUTICS
16.33.1 COMPANY SNAPSHOT
16.33.2 PIPELINE PRODUCT PORTFOLIO
16.33.3 RECENT DEVELOPMENT/NEWS
16.34 VERTEX PHARMACEUTICALS INCORPORATED
16.34.1 COMPANY SNAPSHOT
16.34.2 REVENUE ANALYSIS
16.34.3 PRODUCT PORTFOLIO
16.34.4 RECENT DEVELOPMENT
16.35 VIKING THERAPEUTICS
16.35.1 COMPANY SNAPSHOT
16.35.2 REVENUE ANALYSIS
16.35.3 PIPELINE PRODUCT PORTFOLIO
16.35.4 RECENT DEVELOPMENT
17 QUESTIONNAIRE
18 RELATED REPORTS
표 목록
TABLE 1 NORTH AMERICA INCIDENCE OF CIRRHOSIS BY GENDER (2019)
TABLE 2 TREATMENT ADHERENCE LEVELS IN LIVER DISEASE PATIENTS
TABLE 3 NORTH AMERICA LIVER FIBROSIS TREATMENT MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 4 NORTH AMERICA MEDICATION IN LIVER FIBROSIS TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 5 NORTH AMERICA MEDICATION IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 6 NORTH AMERICA MEDICATION IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 7 NORTH AMERICA ANTIVIRAL AGENTS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 8 NORTH AMERICA ANTIVIRAL AGENTS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 9 NORTH AMERICA ANTIVIRAL AGENTS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 10 NORTH AMERICA ANTIFIBROTIC AGENTS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 11 NORTH AMERICA ANTIFIBROTIC AGENTS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 12 NORTH AMERICA ANTIFIBROTIC AGENTS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 13 NORTH AMERICA ANTI-INFLAMMATORY DRUGS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 14 NORTH AMERICA CORTICOSTEROIDS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 15 NORTH AMERICA CORTICOSTEROIDS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 16 NORTH AMERICA CORTICOSTEROIDS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 17 NORTH AMERICA TUMOR NECROSIS FACTOR (TNF) INHIBITORS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 18 NORTH AMERICA TUMOR NECROSIS FACTOR (TNF) INHIBITORS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 19 NORTH AMERICA TUMOR NECROSIS FACTOR (TNF) INHIBITORS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 20 NORTH AMERICA INTERLEUKIN (IL) INHIBITORS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 21 NORTH AMERICA INTERLEUKIN (IL) INHIBITOR IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 22 NORTH AMERICA INTERLEUKIN (IL) INHIBITOR IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 23 NORTH AMERICA ANTI-INFLAMMATORY DRUGS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 24 NORTH AMERICA IMMUNOSUPPRESSANTS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 25 NORTH AMERICA IMMUNOSUPPRESSANTS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 26 NORTH AMERICA IMMUNOSUPPRESSANTS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 27 NORTH AMERICA MEDICATION IN LIVER FIBROSIS TREATMENT MARKET, BY DRUG STATUS, 2018-2032 (USD THOUSAND)
TABLE 28 NORTH AMERICA MARKETED DRUGS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 29 NORTH AMERICA MEDICATION IN LIVER FIBROSIS TREATMENT MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 30 NORTH AMERICA BRANDED DRUGS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 31 NORTH AMERICA MEDICATION IN LIVER FIBROSIS TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 32 NORTH AMERICA SURGERY/THERAPY IN LIVER FIBROSIS TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 33 NORTH AMERICA SURGERY/THERAPY IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 34 NORTH AMERICA LIVER TRANSPLANTATION IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 35 NORTH AMERICA ENDOSCOPIC & MINIMALLY INVASIVE PROCEDURES IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 36 NORTH AMERICA LIVER ABLATION PROCEDURES IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 37 NORTH AMERICA PARTIAL HEPATECTOMY (LIVER RESECTION) IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 38 NORTH AMERICA CELL-BASED THERAPY IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 39 NORTH AMERICA STEM CELL THERAPY IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 40 NORTH AMERICA GENE THERAPY IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 41 NORTH AMERICA HEPATOCYTE APOPTOSIS INHIBITORS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 42 NORTH AMERICA LIVER FIBROSIS TREATMENT MARKET, BY STAGES, 2018-2032 (USD THOUSAND)
TABLE 43 NORTH AMERICA F2 IN LIVER FIBROSIS TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 44 NORTH AMERICA F1 IN LIVER FIBROSIS TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 45 NORTH AMERICA F3 IN LIVER FIBROSIS TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 46 NORTH AMERICA F4 IN LIVER FIBROSIS TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 47 NORTH AMERICA LIVER FIBROSIS TREATMENT MARKET, BY INDICATION, 2018-2032 (USD THOUSAND)
TABLE 48 NORTH AMERICA NON-ALCOHOLIC STEATOHEPATITIS (NASH) IN LIVER FIBROSIS TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 49 NORTH AMERICA HEPATITIS B & C-INDUCED FIBROSIS IN LIVER FIBROSIS TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 50 NORTH AMERICA HEPATITIS B & C-INDUCED FIBROSIS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 51 NORTH AMERICA ALCOHOLIC LIVER DISEASE (ALD) IN LIVER FIBROSIS TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 52 NORTH AMERICA AUTOIMMUNE LIVER DISEASES IN LIVER FIBROSIS TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 53 NORTH AMERICA AUTOIMMUNE LIVER DISEASES IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 54 NORTH AMERICA GENETIC DISORDERS IN LIVER FIBROSIS TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 55 NORTH AMERICA GENETIC DISORDERS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 56 NORTH AMERICA OTHERS IN LIVER FIBROSIS TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 57 NORTH AMERICA LIVER FIBROSIS TREATMENT MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 58 NORTH AMERICA MALE IN LIVER FIBROSIS TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 59 NORTH AMERICA MALE IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 60 NORTH AMERICA FEMALE IN LIVER FIBROSIS TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 61 NORTH AMERICA FEMALE IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 62 NORTH AMERICA LIVER FIBROSIS TREATMENT MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 63 NORTH AMERICA HOSPITALS IN LIVER FIBROSIS TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 64 NORTH AMERICA HOSPITALS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 65 NORTH AMERICA SPECIALTY CLINICS IN LIVER FIBROSIS TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 66 NORTH AMERICA SPECIALTY CLINICS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 67 NORTH AMERICA CLINICS IN LIVER FIBROSIS TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 68 NORTH AMERICA AMBULATORY AND RESEARCH CENTERS IN LIVER FIBROSIS TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 69 NORTH AMERICA OTHERS IN LIVER FIBROSIS TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 70 NORTH AMERICA LIVER FIBROSIS TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 71 NORTH AMERICA DIRECT TENDER IN LIVER FIBROSIS TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 72 NORTH AMERICA RETAIL SALES IN LIVER FIBROSIS TREATMENT MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 73 NORTH AMERICA RETAIL SALES IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 74 NORTH AMERICA LIVER FIBROSIS TREATMENT MARKET, BY COUNTRY, 2018-2032 (USD THOUSAND)
TABLE 75 NORTH AMERICA LIVER FIBROSIS TREATMENT MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 76 NORTH AMERICA MEDICATION IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 77 NORTH AMERICA MEDICATION IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 78 NORTH AMERICA ANTIVIRAL AGENTS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 79 NORTH AMERICA ANTIVIRAL AGENTS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 80 NORTH AMERICA ANTIVIRAL AGENTS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 81 NORTH AMERICA ANTIFIBROTIC AGENTS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 82 NORTH AMERICA ANTIFIBROTIC AGENTS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 83 NORTH AMERICA ANTIFIBROTIC AGENTS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 84 NORTH AMERICA ANTI-INFLAMMATORY DRUGS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 85 NORTH AMERICA ANTI-INFLAMMATORY DRUGS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 86 NORTH AMERICA CORTICOSTEROIDS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 87 NORTH AMERICA CORTICOSTEROIDS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 88 NORTH AMERICA CORTICOSTEROIDS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 89 NORTH AMERICA TUMOR NECROSIS FACTOR (TNF) INHIBITORS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 90 NORTH AMERICA TUMOR NECROSIS FACTOR (TNF) INHIBITORS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 91 NORTH AMERICA TUMOR NECROSIS FACTOR (TNF) INHIBITORS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 92 NORTH AMERICA INTERLEUKIN (IL) INHIBITORS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 93 NORTH AMERICA INTERLEUKIN (IL) INHIBITOR IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 94 NORTH AMERICA INTERLEUKIN (IL) INHIBITOR IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 95 NORTH AMERICA IMMUNOSUPPRESSANTS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 96 NORTH AMERICA IMMUNOSUPPRESSANTS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 97 NORTH AMERICA IMMUNOSUPPRESSANTS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 98 NORTH AMERICA MEDICATION IN LIVER FIBROSIS TREATMENT MARKET, BY DRUG STATUS, 2018-2032 (USD THOUSAND)
TABLE 99 NORTH AMERICA MARKETED DRUGS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 100 NORTH AMERICA MEDICATION IN LIVER FIBROSIS TREATMENT MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 101 NORTH AMERICA BRANDED DRUGS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 102 NORTH AMERICA MEDICATION IN LIVER FIBROSIS TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 103 NORTH AMERICA SURGERY/THERAPY IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 104 NORTH AMERICA LIVER TRANSPLANTATION IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 105 NORTH AMERICA ENDOSCOPIC & MINIMALLY INVASIVE PROCEDURES IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 106 NORTH AMERICA LIVER ABLATION PROCEDURES IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 107 NORTH AMERICA PARTIAL HEPATECTOMY (LIVER RESECTION) IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 108 NORTH AMERICA CELL-BASED THERAPY IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 109 NORTH AMERICA STEM CELL THERAPY IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 110 NORTH AMERICA GENE THERAPY IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 111 NORTH AMERICA HEPATOCYTE APOPTOSIS INHIBITORS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 112 NORTH AMERICA LIVER FIBROSIS TREATMENT MARKET, BY STAGES, 2018-2032 (USD THOUSAND)
TABLE 113 NORTH AMERICA LIVER FIBROSIS TREATMENT MARKET, BY INDICATION, 2018-2032 (USD THOUSAND)
TABLE 114 NORTH AMERICA HEPATITIS B & C-INDUCED FIBROSIS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 115 NORTH AMERICA AUTOIMMUNE LIVER DISEASES IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 116 NORTH AMERICA GENETIC DISORDERS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 117 NORTH AMERICA LIVER FIBROSIS TREATMENT MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 118 NORTH AMERICA MALE IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 119 NORTH AMERICA FEMALE IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 120 NORTH AMERICA LIVER FIBROSIS TREATMENT MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 121 NORTH AMERICA HOSPITALS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 122 NORTH AMERICA SPECIALTY CLINICS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 123 NORTH AMERICA LIVER FIBROSIS TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 124 NORTH AMERICA RETAIL SALES IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 125 U.S. LIVER FIBROSIS TREATMENT MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 126 U.S. MEDICATION IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 127 U.S. MEDICATION IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 128 U.S. ANTIVIRAL AGENTS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 129 U.S. ANTIVIRAL AGENTS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 130 U.S. ANTIVIRAL AGENTS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 131 U.S. ANTIFIBROTIC AGENTS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 132 U.S. ANTIFIBROTIC AGENTS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 133 U.S. ANTIFIBROTIC AGENTS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 134 U.S. ANTI-INFLAMMATORY DRUGS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 135 U.S. ANTI-INFLAMMATORY DRUGS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 136 U.S. CORTICOSTEROIDS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 137 U.S. CORTICOSTEROIDS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 138 U.S. CORTICOSTEROIDS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 139 U.S. TUMOR NECROSIS FACTOR (TNF) INHIBITORS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 140 U.S. TUMOR NECROSIS FACTOR (TNF) INHIBITORS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 141 U.S. TUMOR NECROSIS FACTOR (TNF) INHIBITORS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 142 U.S. INTERLEUKIN (IL) INHIBITORS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 143 U.S. INTERLEUKIN (IL) INHIBITOR IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 144 U.S. INTERLEUKIN (IL) INHIBITOR IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 145 U.S. IMMUNOSUPPRESSANTS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 146 U.S. IMMUNOSUPPRESSANTS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 147 U.S. IMMUNOSUPPRESSANTS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 148 U.S. MEDICATION IN LIVER FIBROSIS TREATMENT MARKET, BY DRUG STATUS, 2018-2032 (USD THOUSAND)
TABLE 149 U.S. MARKETED DRUGS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 150 U.S. MEDICATION IN LIVER FIBROSIS TREATMENT MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 151 U.S. BRANDED DRUGS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 152 U.S. MEDICATION IN LIVER FIBROSIS TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 153 U.S. SURGERY/THERAPY IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 154 U.S. LIVER TRANSPLANTATION IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 155 U.S. ENDOSCOPIC & MINIMALLY INVASIVE PROCEDURES IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 156 U.S. LIVER ABLATION PROCEDURES IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 157 U.S. PARTIAL HEPATECTOMY (LIVER RESECTION) IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 158 U.S. CELL-BASED THERAPY IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 159 U.S. STEM CELL THERAPY IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 160 U.S. GENE THERAPY IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 161 U.S. HEPATOCYTE APOPTOSIS INHIBITORS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 162 U.S. LIVER FIBROSIS TREATMENT MARKET, BY STAGES, 2018-2032 (USD THOUSAND)
TABLE 163 U.S. LIVER FIBROSIS TREATMENT MARKET, BY INDICATION, 2018-2032 (USD THOUSAND)
TABLE 164 U.S. HEPATITIS B & C-INDUCED FIBROSIS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 165 U.S. AUTOIMMUNE LIVER DISEASES IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 166 U.S. GENETIC DISORDERS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 167 U.S. LIVER FIBROSIS TREATMENT MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 168 U.S. MALE IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 169 U.S. FEMALE IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 170 U.S. LIVER FIBROSIS TREATMENT MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 171 U.S. HOSPITALS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 172 U.S. SPECIALTY CLINICS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 173 U.S. LIVER FIBROSIS TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 174 U.S. RETAIL SALES IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 175 CANADA LIVER FIBROSIS TREATMENT MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 176 CANADA MEDICATION IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 177 CANADA MEDICATION IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 178 CANADA ANTIVIRAL AGENTS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 179 CANADA ANTIVIRAL AGENTS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 180 CANADA ANTIVIRAL AGENTS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 181 CANADA ANTIFIBROTIC AGENTS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 182 CANADA ANTIFIBROTIC AGENTS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 183 CANADA ANTIFIBROTIC AGENTS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 184 CANADA ANTI-INFLAMMATORY DRUGS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 185 CANADA ANTI-INFLAMMATORY DRUGS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 186 CANADA CORTICOSTEROIDS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 187 CANADA CORTICOSTEROIDS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 188 CANADA CORTICOSTEROIDS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 189 CANADA TUMOR NECROSIS FACTOR (TNF) INHIBITORS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 190 CANADA TUMOR NECROSIS FACTOR (TNF) INHIBITORS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 191 CANADA TUMOR NECROSIS FACTOR (TNF) INHIBITORS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 192 CANADA INTERLEUKIN (IL) INHIBITORS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 193 CANADA INTERLEUKIN (IL) INHIBITOR IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 194 CANADA INTERLEUKIN (IL) INHIBITOR IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 195 CANADA IMMUNOSUPPRESSANTS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 196 CANADA IMMUNOSUPPRESSANTS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 197 CANADA IMMUNOSUPPRESSANTS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 198 CANADA MEDICATION IN LIVER FIBROSIS TREATMENT MARKET, BY DRUG STATUS, 2018-2032 (USD THOUSAND)
TABLE 199 CANADA MARKETED DRUGS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 200 CANADA MEDICATION IN LIVER FIBROSIS TREATMENT MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 201 CANADA BRANDED DRUGS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 202 CANADA MEDICATION IN LIVER FIBROSIS TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 203 CANADA SURGERY/THERAPY IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 204 CANADA LIVER TRANSPLANTATION IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 205 CANADA ENDOSCOPIC & MINIMALLY INVASIVE PROCEDURES IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 206 CANADA LIVER ABLATION PROCEDURES IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 207 CANADA PARTIAL HEPATECTOMY (LIVER RESECTION) IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 208 CANADA CELL-BASED THERAPY IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 209 CANADA STEM CELL THERAPY IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 210 CANADA GENE THERAPY IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 211 CANADA HEPATOCYTE APOPTOSIS INHIBITORS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 212 CANADA LIVER FIBROSIS TREATMENT MARKET, BY STAGES, 2018-2032 (USD THOUSAND)
TABLE 213 CANADA LIVER FIBROSIS TREATMENT MARKET, BY INDICATION, 2018-2032 (USD THOUSAND)
TABLE 214 CANADA HEPATITIS B & C-INDUCED FIBROSIS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 215 CANADA AUTOIMMUNE LIVER DISEASES IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 216 CANADA GENETIC DISORDERS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 217 CANADA LIVER FIBROSIS TREATMENT MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 218 CANADA MALE IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 219 CANADA FEMALE IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 220 CANADA LIVER FIBROSIS TREATMENT MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 221 CANADA HOSPITALS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 222 CANADA SPECIALTY CLINICS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 223 CANADA LIVER FIBROSIS TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 224 CANADA RETAIL SALES IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 225 MEXICO LIVER FIBROSIS TREATMENT MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 226 MEXICO MEDICATION IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 227 MEXICO MEDICATION IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 228 MEXICO ANTIVIRAL AGENTS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 229 MEXICO ANTIVIRAL AGENTS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 230 MEXICO ANTIVIRAL AGENTS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 231 MEXICO ANTIFIBROTIC AGENTS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 232 MEXICO ANTIFIBROTIC AGENTS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 233 MEXICO ANTIFIBROTIC AGENTS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 234 MEXICO ANTI-INFLAMMATORY DRUGS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 235 MEXICO ANTI-INFLAMMATORY DRUGS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 236 MEXICO CORTICOSTEROIDS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 237 MEXICO CORTICOSTEROIDS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 238 MEXICO CORTICOSTEROIDS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 239 MEXICO TUMOR NECROSIS FACTOR (TNF) INHIBITORS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 240 MEXICO TUMOR NECROSIS FACTOR (TNF) INHIBITORS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 241 MEXICO TUMOR NECROSIS FACTOR (TNF) INHIBITORS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 242 MEXICO INTERLEUKIN (IL) INHIBITORS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 243 MEXICO INTERLEUKIN (IL) INHIBITOR IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 244 MEXICO INTERLEUKIN (IL) INHIBITOR IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 245 MEXICO IMMUNOSUPPRESSANTS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 246 MEXICO IMMUNOSUPPRESSANTS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (VOLUME IN THOUSAND UNITS)
TABLE 247 MEXICO IMMUNOSUPPRESSANTS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (ASP IN USD/UNITS)
TABLE 248 MEXICO MEDICATION IN LIVER FIBROSIS TREATMENT MARKET, BY DRUG STATUS, 2018-2032 (USD THOUSAND)
TABLE 249 MEXICO MARKETED DRUGS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 250 MEXICO MEDICATION IN LIVER FIBROSIS TREATMENT MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 251 MEXICO BRANDED DRUGS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 252 MEXICO MEDICATION IN LIVER FIBROSIS TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 253 MEXICO SURGERY/THERAPY IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 254 MEXICO LIVER TRANSPLANTATION IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 255 MEXICO ENDOSCOPIC & MINIMALLY INVASIVE PROCEDURES IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 256 MEXICO LIVER ABLATION PROCEDURES IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 257 MEXICO PARTIAL HEPATECTOMY (LIVER RESECTION) IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 258 MEXICO CELL-BASED THERAPY IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 259 MEXICO STEM CELL THERAPY IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 260 MEXICO GENE THERAPY IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 261 MEXICO HEPATOCYTE APOPTOSIS INHIBITORS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 262 MEXICO LIVER FIBROSIS TREATMENT MARKET, BY STAGES, 2018-2032 (USD THOUSAND)
TABLE 263 MEXICO LIVER FIBROSIS TREATMENT MARKET, BY INDICATION, 2018-2032 (USD THOUSAND)
TABLE 264 MEXICO HEPATITIS B & C-INDUCED FIBROSIS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 265 MEXICO AUTOIMMUNE LIVER DISEASES IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 266 MEXICO GENETIC DISORDERS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 267 MEXICO LIVER FIBROSIS TREATMENT MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 268 MEXICO MALE IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 269 MEXICO FEMALE IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 270 MEXICO LIVER FIBROSIS TREATMENT MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 271 MEXICO HOSPITALS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 272 MEXICO SPECIALTY CLINICS IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 273 MEXICO LIVER FIBROSIS TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 274 MEXICO RETAIL SALES IN LIVER FIBROSIS TREATMENT MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
그림 목록
FIGURE 1 NORTH AMERICA LIVER FIBROSIS TREATMENT MARKET: SEGMENTATION
FIGURE 2 NORTH AMERICA LIVER FIBROSIS TREATMENT MARKET: DATA TRIANGULATION
FIGURE 3 NORTH AMERICA LIVER FIBROSIS TREATMENT MARKET: DROC ANALYSIS
FIGURE 4 NORTH AMERICA LIVER FIBROSIS TREATMENT MARKET: REGIONAL MARKET ANALYSIS
FIGURE 5 NORTH AMERICA LIVER FIBROSIS TREATMENT MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 NORTH AMERICA LIVER FIBROSIS TREATMENT MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 NORTH AMERICA LIVER FIBROSIS TREATMENT MARKET: DBMR MARKET POSITION GRID
FIGURE 8 NORTH AMERICA LIVER FIBROSIS TREATMENT MARKET: MARKET END USER COVERAGE GRID
FIGURE 9 NORTH AMERICA LIVER FIBROSIS TREATMENT MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 NORTH AMERICA LIVER FIBROSIS TREATMENT MARKET: SEGMENTATION
FIGURE 11 INCREASING PREVALENCE OF LIVER DISEASES IS EXPECTED TO DRIVE THE GROWTH OF NORTH AMERICA LIVER FIBROSIS TREATMENT MARKET FROM 2025 TO 2032
FIGURE 12 THE MEDICATION SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE NORTH AMERICA LIVER FIBROSIS TREATMENT MARKET IN 2025-2032
FIGURE 13 TWO SEGMENTS COMPRISE THE NORTH AMERICA LIVER FIBROSIS TREATMENT MARKET, BY TREATMENT TYPE
FIGURE 14 NORTH AMERICA LIVER FIBROSIS TREATMENT MARKET: EXECUTIVE SUMMARY
FIGURE 15 STRATEGIC DECISIONS
FIGURE 16 DRIVERS, RESTRAINTS, OPPORTUNITIES AND CHALLENGES OF NORTH AMERICA LIVER FIBROSIS TREATMENT MARKET
FIGURE 17 NORTH AMERICA LIVER FIBROSIS TREATMENT MARKET: BY TREATMENT TYPE, 2024
FIGURE 18 NORTH AMERICA LIVER FIBROSIS TREATMENT MARKET: BY TREATMENT TYPE, 2025-2032 (USD THOUSAND)
FIGURE 19 NORTH AMERICA LIVER FIBROSIS TREATMENT MARKET: BY TREATMENT TYPE, CAGR (2025-2032)
FIGURE 20 NORTH AMERICA LIVER FIBROSIS TREATMENT MARKET: BY TREATMENT TYPE, LIFELINE CURVE
FIGURE 21 NORTH AMERICA LIVER FIBROSIS TREATMENT MARKET: BY STAGES, 2024
FIGURE 22 NORTH AMERICA LIVER FIBROSIS TREATMENT MARKET: BY STAGES, 2025-2032 (USD THOUSAND)
FIGURE 23 NORTH AMERICA LIVER FIBROSIS TREATMENT MARKET: BY STAGES, CAGR (2025-2032)
FIGURE 24 NORTH AMERICA LIVER FIBROSIS TREATMENT MARKET: BY STAGES, LIFELINE CURVE
FIGURE 25 NORTH AMERICA LIVER FIBROSIS TREATMENT MARKET: BY INDICATION, 2024
FIGURE 26 NORTH AMERICA LIVER FIBROSIS TREATMENT MARKET: BY INDICATION, 2025-2032 (USD THOUSAND)
FIGURE 27 NORTH AMERICA LIVER FIBROSIS TREATMENT MARKET: BY INDICATION, CAGR (2025-2032)
FIGURE 28 NORTH AMERICA LIVER FIBROSIS TREATMENT MARKET: BY INDICATION, LIFELINE CURVE
FIGURE 29 NORTH AMERICA LIVER FIBROSIS TREATMENT MARKET: BY GENDER, 2024
FIGURE 30 NORTH AMERICA LIVER FIBROSIS TREATMENT MARKET: BY GENDER, 2025-2032 (USD THOUSAND)
FIGURE 31 NORTH AMERICA LIVER FIBROSIS TREATMENT MARKET: BY GENDER, CAGR (2025-2032)
FIGURE 32 NORTH AMERICA LIVER FIBROSIS TREATMENT MARKET: BY GENDER, LIFELINE CURVE
FIGURE 33 NORTH AMERICA LIVER FIBROSIS TREATMENT MARKET: BY END USER, 2024
FIGURE 34 NORTH AMERICA LIVER FIBROSIS TREATMENT MARKET: BY END USER, 2025-2032 (USD THOUSAND)
FIGURE 35 NORTH AMERICA LIVER FIBROSIS TREATMENT MARKET: BY END USER, CAGR (2025-2032)
FIGURE 36 NORTH AMERICA LIVER FIBROSIS TREATMENT MARKET: BY END USER, LIFELINE CURVE
FIGURE 37 NORTH AMERICA LIVER FIBROSIS TREATMENT MARKET: BY DISTRIBUTION CHANNEL 2024
FIGURE 38 NORTH AMERICA LIVER FIBROSIS TREATMENT MARKET: BY DISTRIBUTION CHANNEL, 2025-2032 (USD THOUSAND)
FIGURE 39 NORTH AMERICA LIVER FIBROSIS TREATMENT MARKET: BY DISTRIBUTION CHANNEL, CAGR (2025-2032)
FIGURE 40 NORTH AMERICA LIVER FIBROSIS TREATMENT MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 41 NORTH AMERICA LIVER FIBROSIS TREATMENT MARKET: SNAPSHOT (2024)
FIGURE 42 NORTH AMERICA LIVER FIBROSIS TREATMENT MARKET: COMPANY SHARE 2024 (%)

연구 방법론
데이터 수집 및 기준 연도 분석은 대규모 샘플 크기의 데이터 수집 모듈을 사용하여 수행됩니다. 이 단계에는 다양한 소스와 전략을 통해 시장 정보 또는 관련 데이터를 얻는 것이 포함됩니다. 여기에는 과거에 수집한 모든 데이터를 미리 검토하고 계획하는 것이 포함됩니다. 또한 다양한 정보 소스에서 발견되는 정보 불일치를 검토하는 것도 포함됩니다. 시장 데이터는 시장 통계 및 일관된 모델을 사용하여 분석하고 추정합니다. 또한 시장 점유율 분석 및 주요 추세 분석은 시장 보고서의 주요 성공 요인입니다. 자세한 내용은 분석가에게 전화를 요청하거나 문의 사항을 드롭하세요.
DBMR 연구팀에서 사용하는 주요 연구 방법론은 데이터 마이닝, 시장에 대한 데이터 변수의 영향 분석 및 주요(산업 전문가) 검증을 포함하는 데이터 삼각 측량입니다. 데이터 모델에는 공급업체 포지셔닝 그리드, 시장 타임라인 분석, 시장 개요 및 가이드, 회사 포지셔닝 그리드, 특허 분석, 가격 분석, 회사 시장 점유율 분석, 측정 기준, 글로벌 대 지역 및 공급업체 점유율 분석이 포함됩니다. 연구 방법론에 대해 자세히 알아보려면 문의를 통해 업계 전문가에게 문의하세요.
사용자 정의 가능
Data Bridge Market Research는 고급 형성 연구 분야의 선두 주자입니다. 저희는 기존 및 신규 고객에게 목표에 맞는 데이터와 분석을 제공하는 데 자부심을 느낍니다. 보고서는 추가 국가에 대한 시장 이해(국가 목록 요청), 임상 시험 결과 데이터, 문헌 검토, 재생 시장 및 제품 기반 분석을 포함하도록 사용자 정의할 수 있습니다. 기술 기반 분석에서 시장 포트폴리오 전략에 이르기까지 타겟 경쟁업체의 시장 분석을 분석할 수 있습니다. 귀하가 원하는 형식과 데이터 스타일로 필요한 만큼 많은 경쟁자를 추가할 수 있습니다. 저희 분석가 팀은 또한 원시 엑셀 파일 피벗 테이블(팩트북)로 데이터를 제공하거나 보고서에서 사용 가능한 데이터 세트에서 프레젠테이션을 만드는 데 도움을 줄 수 있습니다.