隨機化和試驗供應管理 (RTSM) 市場擁有即時藥物分配、患者隨機化和庫存管理等功能。占主導地位的領域是製藥業,其中 RTSM 簡化了複雜的臨床試驗。它確保了正確的患者分配、藥物供應和數據的準確性。 RTSM 還支援自適應試驗設計,從而降低成本和縮短時間。隨著藥物研究的不斷發展,RTSM 市場的重要性不斷提升,提高了藥物開發臨床試驗的效率和準確性。
Data Bridge Market Research 分析稱,亞太地區隨機化和試驗供應管理 (RTSM) 市場規模在 2022 年為 3.6208 億美元,預計到 2030 年將達到 10.8471 億美元,預計在 2023 年至 2030 年的預測期內複合年增長率為 14.7%。由隨機化和試驗供應管理 (RTSM) 推動的自適應試驗設計允許根據新興數據即時調整臨床試驗,優化資源配置,並增強應對製藥業藥物開發動態特性所需的適應性。
研究的主要發現
臨床試驗的複雜性不斷增加預計將推動市場成長率
隨著臨床試驗的複雜性不斷增加,通常涉及大量參與者和適應性研究設計,對高效隨機化和試驗供應管理 (RTSM) 系統的需求也在增加。這些系統確保了精確的患者隨機化和簡化的藥物供應管理,減少了錯誤和後勤挑戰。在藥物開發不斷發展的時代,RTSM 在確保試驗順利進行、維護數據完整性和適應不斷變化的藥物研究情況方面發揮關鍵作用。
報告範圍和市場細分
報告指標
|
細節
|
預測期
|
2023年至2030年
|
基準年
|
2022
|
歷史歲月
|
2021(可自訂為2015-2020)
|
定量單位
|
收入(百萬美元)、銷售(單位)、定價(美元)
|
涵蓋的領域
|
組件(軟體、服務)、交付模式(授權企業(本地)、基於雲端的 (SaaS)、基於 Web 的(按需)、應用程式(情境規劃和預測、聚合/批次規劃、研究建構、隨機化、藥物分配、重新預測和優化、銷毀和協調、資料庫鎖定、庫存管理、其他)、臨床試驗類型(治療試驗、預防試驗、預防試驗和協調、資料庫鎖定、庫存管理、其他)、臨床試驗類型(治療試驗、預防試驗、預防試驗階段期臨床試驗和上市前/上市後)、治療領域(腫瘤學、心血管疾病和循環系統疾病、傳染病、消化系統疾病、肌肉骨骼疾病、神經系統疾病、內分泌和代謝疾病、精神健康和行為障礙、血液疾病、呼吸系統疾病、其他)、最終用戶(製藥和生物製藥公司、合約研究組織、醫療分銷商、醫院、學術研究機構、政府機構、其他機構
|
覆蓋國家
|
亞太地區 (APAC) 中的中國、日本、印度、韓國、新加坡、馬來西亞、澳洲、泰國、印尼、菲律賓、亞太地區 (APAC) 其他地區。
|
涵蓋的市場參與者
|
MEDICAL Information Technology Inc.(美國)、SAP(德國)、CPSI(美國)、Meta(美國)、Elinext(美國)、EPIC Systems Corporation(美國)、INFOR(美國)、Cognizant(美國)、Oracle(美國)、Jag products LLC(美國)、Allscripts Corporation LLC(美國)、Otumk) Healthcare(美國)、Koninklijke Philips NV(荷蘭)、athenahealth(美國)、eClinicalWorks(美國)
|
報告涵蓋的數據點
|
除了對市場價值、成長率、細分、地理覆蓋範圍和主要參與者等市場情景的洞察之外,Data Bridge Market Research 策劃的市場報告還包括深度專家分析、患者流行病學、管道分析、定價分析和監管框架。
|
細分分析:
亞太隨機化和試驗供應管理 (RTSM) 市場根據組件、交付模式、應用、臨床試驗類型、臨床試驗階段、治療領域、最終用戶和分銷管道進行細分。
- 根據組成部分,亞太隨機化和試驗供應管理 (RTSM) 市場分為軟體和服務。
- 根據交付模式,亞太隨機化和試驗供應管理 (RTSM) 市場細分為授權企業(內部部署)、基於雲端(SaaS)、基於網路(按需)。
- 根據應用,亞太隨機化和試驗供應管理 (RTSM) 市場細分為情境規劃和預測、聚合/批次規劃、研究建構、隨機化、藥物分配、重新預測和優化、銷毀和核對、資料庫鎖定、庫存管理等。
- 根據臨床試驗類型,亞太隨機化和試驗供應管理 (RTSM) 市場分為治療試驗、預防試驗、篩檢試驗和支持性護理試驗。
- 根據臨床試驗階段,亞太隨機化和試驗供應管理 (RTSM) 市場分為早期、I 期臨床試驗、II 期臨床試驗、III 期臨床試驗、IV 期臨床試驗以及批准期間/批准後。
- 根據治療領域,亞太隨機化和試驗供應管理(RTSM)市場細分為腫瘤學、心血管疾病和循環系統疾病、傳染病、消化系統疾病、肌肉骨骼疾病、神經系統疾病、內分泌和代謝疾病、精神健康和行為障礙、血液疾病、呼吸系統疾病等。
- 根據最終用戶,亞太隨機化和試驗供應管理 (RTSM) 市場細分為製藥和生物製藥公司、合約研究組織、醫療器材製造商、醫院、學術研究機構、政府機構等。
- 根據分銷管道,亞太隨機化和試驗供應管理 (RTSM) 市場分為直銷和第三方分銷商。
主要參與者
Data Bridge Market Research 將以下公司認定為亞太隨機化和試驗供應管理 (RTSM) 市場的參與者,亞太隨機化和試驗供應管理 (RTSM) 市場包括 MEDICAL Information Technology Inc.(美國)、SAP(德國)、CPSI(美國)、Meta(美國)、Elinext(美國)、EPIC Systems Corporation(美國)、INFOR(美國)、EPIC Systems Corporation(美國)、EPIC Systems Corporation(美國)、EPIC(EPIC)。
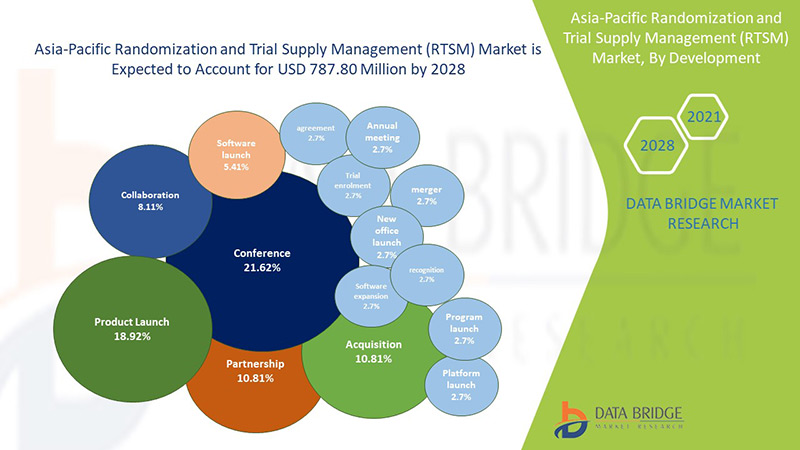
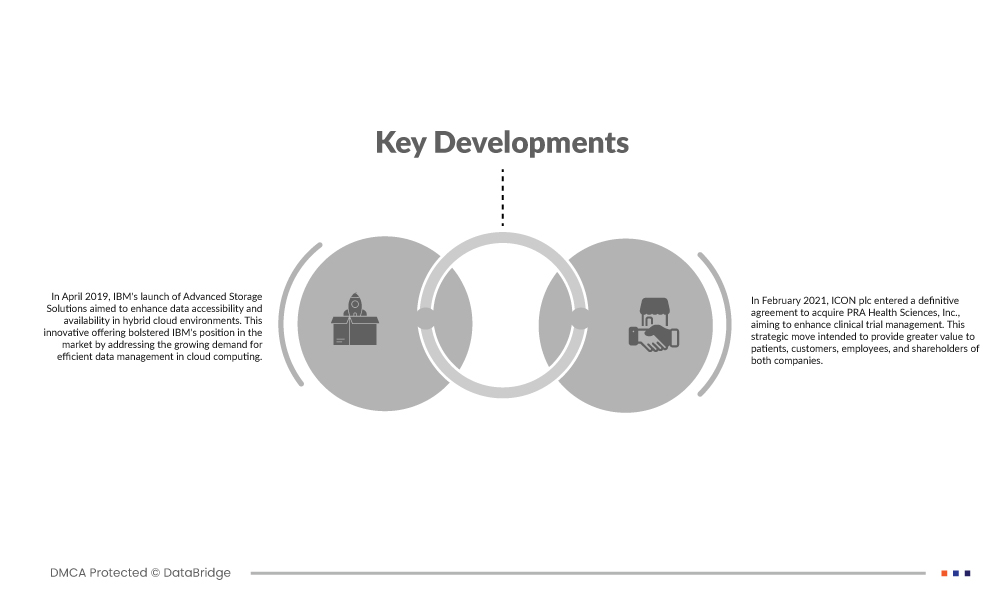
市場發展
- 2019年4月,IBM推出進階儲存解決方案,旨在增強混合雲環境中的資料可存取性和可用性。這項創新產品滿足了雲端運算中日益增長的高效資料管理需求,鞏固了 IBM 在市場上的地位。結果,它吸引了新客戶並擴大了公司的市場份額,從而增加了收入。此舉符合 IBM 為不斷發展的 IT 格局提供尖端解決方案的策略。
- 2021 年 2 月,ICON plc 達成最終協議,收購 PRA Health Sciences, Inc.,旨在加強臨床試驗管理。這項策略性措施旨在為兩家公司的患者、客戶、員工和股東提供更大的價值。此次收購整合了臨床研究產業的資源、專業知識和能力,從而能夠提供更有效率、更全面的解決方案來支持創新醫療療法和治療方法的開發。
區域分析
從地理上看,亞太隨機化和試驗供應管理 (RTSM) 市場報告涵蓋的國家包括亞太地區的中國、日本、印度、韓國、新加坡、馬來西亞、澳洲、泰國、印尼、菲律賓、亞太地區其他地區 (APAC)。
根據 Data Bridge 市場研究分析:
預計2023-2030 年預測期內,日本將主導亞太隨機化與試驗供應管理 (RTSM) 市場
由於醫療保健的進步,日本有望引領亞太地區 (APAC) 隨機化和試驗供應管理 (RTSM) 市場。該國致力於尖端醫學研究和臨床試驗,加上強大的醫療保健基礎設施,使其成為 RTSM 技術採用領域的主導者。日本對精準醫療的關注及其作為醫藥創新中心的角色使其成為亞太地區 RTSM 市場成長的主要驅動力。
有關亞太隨機化和試驗供應管理 (RTSM) 市場報告的更多詳細信息,請點擊此處 - https://www.databridgemarketresearch.com/reports/asia-pacific-randomization-and-trial-supply-management-rtsm-market