Gastric cancer treatments encompass a range of approaches, including surgery, chemotherapy, targeted therapies, and immunotherapies. Surgery removes cancerous tissues, while chemotherapy targets rapidly dividing cells. Targeted therapies focus on specific molecular abnormalities in cancer cells, minimizing damage to healthy tissues. Immunotherapies boost the immune system's ability to recognize and combat cancer cells. These treatments collectively aim to eradicate or control gastric cancer, enhancing patient survival rates and quality of life by tailoring approaches to individual patients' needs.
Access Full Report @ https://www.databridgemarketresearch.com/reports/central-america-gastric-cancer-treatment-market
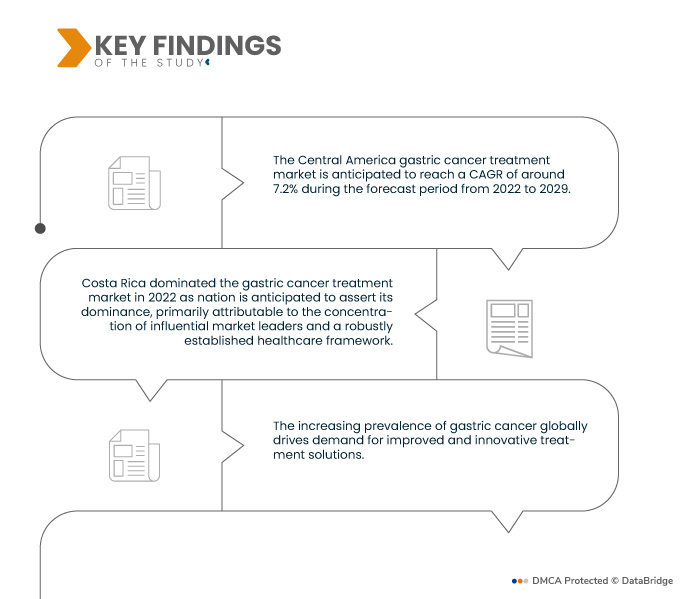
Data Bridge Market Research analyses that the Central America Gastric Cancer Treatment Market is registering a CAGR of 7.2% during the forecast period of 2022 to 2029. Continuous advancements in targeted therapies and immunotherapies are reshaping gastric cancer treatment. These innovative approaches precisely target cancer cells or enhance the immune system's response, leading to improved outcomes.
Key Findings of the Study
Early diagnosis is expected to drive the market's growth rate
Increasing awareness about gastric cancer symptoms and risk factors prompts individuals to seek medical attention earlier. With advanced diagnostic techniques such as endoscopy, imaging, and molecular tests, healthcare providers can detect gastric cancer at its initial stages. Early detection allows for more effective treatment options and improved prognosis. By identifying the disease before it progresses, patients have a higher chance of successful interventions, leading to enhanced treatment success rates and better overall outcomes.
Report Scope and Market Segmentation
|
Report Metric
|
Details
|
|
Forecast Period
|
2022 to 2029
|
|
Base Year
|
2021
|
|
Historic Years
|
2020 (Customizable to 2014-2019)
|
|
Segments Covered
|
Type (Adenocarcinoma, gastrointestinal stromal tumor, Carcinoid Tumor, Lymphoma, and Others), Stages (Stage III, Stage II, Stage IV, Stage I, and Others), Treatment (Chemotherapy, Targeted Therapy, Immune Checkpoint Inhibitors, and Others), Route of Administration (Parenteral, Oral and Others), End-Users (Hospitals, Specialty Clinics, Research and Academic Institutes and Others), Distribution Channel (Hospital Pharmacy, Retail Pharmacy, and Others)
|
|
Countries Covered
|
Costa Rica, Guatemala, Panama, Dominican Republic, and Rest of Central America
|
|
Market Players Covered
|
F. Hoffmann-La Roche Ltd. (Switzerland), Merck and Co., Inc. (U.S.), Pfizer Inc. (U.S.), Teva Pharmaceutical Industries Ltd. (Israel), AstraZeneca (U.K.), Bayer AG (Germany), Novartis AG (Switzerland), Bristol-Myers Squibb Company (U.S.), Eli Lilly and Company (U.S.), Laboratorio Varifarma SA (Argentina), Seven pharma (Japan), Janssen Global Services, LLC (A subsidiary of Johnson and Johnson Services, Inc.) (U.S.), Sanofi (France)
|
|
Data Points Covered in the Report
|
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework
|
Segment Analysis:
The Central America gastric cancer treatment market is segmented on the basis of type, stage, treatment, route of administration, end-users, and distribution channel.
- On the basis of type, the market is segmented into adenocarcinoma, lymphoma, gastrointestinal stromal tumor, carcinoid tumor, and others.
- On the basis of stage, the market is segmented into stage I, stage II, stage III, stage IV, and others.
- On the basis of treatment, the market is segmented into chemotherapy, targeted therapy, immunotherapy, and others.
- On the basis of route of administration, the market is segmented into oral and parenteral.
- On the basis of end user, the Central America gastric cancer treatment market is segmented into hospitals, specialty clinics, research and academic institutes, and others.
- On the basis of distribution channel into retail pharmacy, hospital pharmacy, and others.
Major Players
Data Bridge Market Research recognizes the following companies as the major Central America gastric cancer treatment market players in Central America gastric cancer treatment market are F.Hoffmann-La Roche Ltd. (Switzerland), Merck and Co., Inc. (U.S.), Pfizer Inc. (U.S.), Teva Pharmaceutical Industries Ltd. (Israel), AstraZeneca (U.K.), Bayer AG (Germany), Novartis AG (Switzerland), Bristol-Myers Squibb Company (U.S.), Eli Lilly and Company (U.S.)
Market Developments
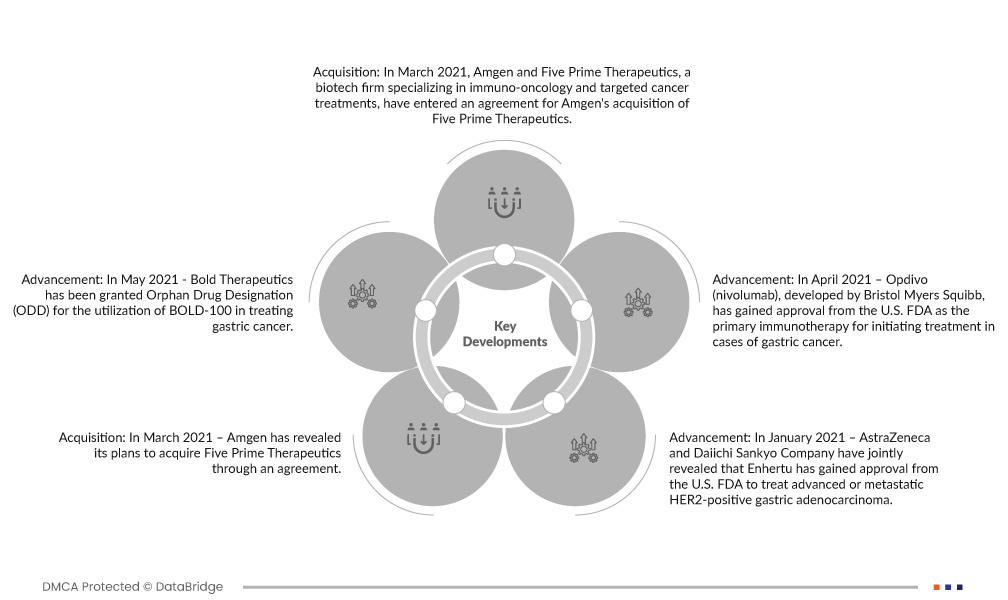
- In March 2021, Amgen and Five Prime Therapeutics, a biotech firm specializing in immuno-oncology and targeted cancer treatments, have entered an agreement for Amgen's acquisition of Five Prime Therapeutics. This acquisition encompasses Bemarituzumab, an advanced Phase 3 program aimed at treating gastric cancer.
- In May 2021, Bold Therapeutics has been granted Orphan Drug Designation (ODD) for the utilization of BOLD-100 in treating gastric cancer. BOLD-100 represents a pioneering small molecule therapeutics, leveraging ruthenium, and is recognized for its novel approach.
- In April 2021, Opdivo (nivolumab), developed by Bristol Myers Squibb, has gained approval from the U.S. FDA as the primary immunotherapy for initiating treatment in cases of gastric cancer This marks a significant milestone, positioning Opdivo as the foremost immunotherapeutic option in the frontline management of this condition.
- In March 2021, Amgen revealed its plans to acquire Five Prime Therapeutics through an agreement. This strategic move involves the integration of Five Prime's pipeline asset, an anti-FGFR2b antibody targeting gastric cancer, into Amgen's existing oncology portfolio.
- In January 2021, AstraZeneca and Daiichi Sankyo Company have jointly revealed that Enhertu has gained approval from the U.S. FDA to treat advanced or metastatic HER2-positive gastric adenocarcinoma. This collaboration marks a significant step forward in addressing this specific form of cancer.
Regional Analysis
Geographically, the countries covered in the Gastric cancer treatment market report are Costa Rica, Guatemala, Panama, Dominican Republic, and Rest of Central America
As per Data Bridge Market Research analysis:
Costa Rica is the dominant region in the gastric cancer treatment market during the forecast period 2022 - 2029
In 2022, Costa Rica dominated the gastric cancer treatment market due to the interplay of industry leaders and a robust medical infrastructure creates a fertile ground for growth and innovation in the healthcare sector. This synergy fosters an environment that supports continuous advancement, propelling the nation's prominence in the field. With established giants driving progress and a well-organized healthcare framework in place, the country is poised to maintain its upward trajectory, solidifying its position as a key player in the foreseeable future.
For more detailed information about the gastric cancer treatment market report, click here – https://www.databridgemarketresearch.com/reports/central-america-gastric-cancer-treatment-market