Европейский рынок электронных клинических решений относится к внедрению электронных технологий и программных систем в сфере здравоохранения для управления и проведения клинических испытаний и исследований. Эти решения охватывают различные электронные системы и платформы, включая электронный сбор данных, управление клиническими испытаниями, результаты, сообщаемые пациентами, и аналитику данных. Они используются фармацевтическими компаниями, организациями по контрактным исследованиям и академическими учреждениями для оптимизации процесса клинических испытаний, повышения точности данных и ускорения разработки лекарств. Будучи критически важным сегментом сектора медицинских технологий, европейский рынок электронных клинических решений играет решающую роль в продвижении медицинских исследований и улучшении результатов для пациентов.
Доступ к полному отчету по адресу https://www.databridgemarketresearch.com/reports/europe-eclinical-solutions-market
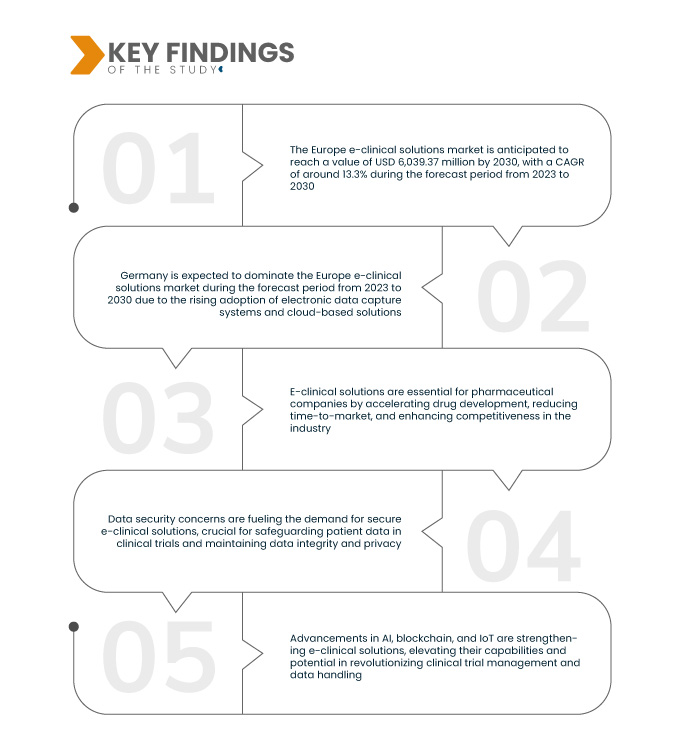
Data Bridge Market Research анализирует, что ожидается, что рынок европейских электронных клинических решений будет расти в среднем на 13,3% в течение прогнозируемого периода с 2023 по 2030 год и, как ожидается, достигнет 6 039,37 млн долларов США к 2030 году с 2 224,09 млн долларов США к 2022 году. Переход к ориентированным на пациента испытаниям и удаленному мониторингу требует адаптируемых электронных клинических решений. Эта эволюция подчеркивает жизненно важную роль технологий в улучшении опыта пациентов, обеспечении доступа к данным в реальном времени и обеспечении эффективных медицинских испытаний, что соответствует фокусу отрасли на персонализированных и удаленных подходах к медицинским исследованиям.
Основные выводы исследования
Ожидается, что глобальное сотрудничество в области клинических исследований будет способствовать темпам роста рынка.
В сфере клинических исследований глобальное сотрудничество стало ключевым. Международные совместные усилия требуют передовых электронных клинических систем, способных управлять разнообразными данными из разных регионов, обеспечивая при этом единообразие и согласованность. Эти сложные решения облегчают бесперебойную коммуникацию, обмен данными и анализ через границы, способствуя эффективному исследовательскому партнерству. В эпоху, когда преобладают трансграничные исследования, эти системы являются основополагающими, позволяя исследователям по всему миру работать слаженно, стимулируя инновации и прогресс в исследованиях в области здравоохранения. Область применения отчета и сегментация рынка
Отчет Метрика
|
Подробности
|
Прогнозируемый период
|
2023-2030
|
Базовый год
|
2022
|
Исторические годы
|
2021 (Можно настроить на 2015-2020)
|
Количественные единицы
|
Доход в млн. долл. США, объемы в единицах, цены в долл. США
|
Охваченные сегменты
|
Продукт (электронные системы сбора данных и управления данными клинических испытаний, системы управления клиническими испытаниями, платформы клинической аналитики, медицинские записи для координации ухода (CCMR), рандомизация и управление поставками для испытаний, платформы интеграции клинических данных, электронные решения для оценки клинических результатов, решения по безопасности, электронные системы основных файлов испытаний, решения по управлению нормативной информацией и другие), режим доставки (решения, размещенные в Интернете (по запросу), лицензированные корпоративные (локальные) решения и облачные (SAAS) решения), фаза клинического испытания (фаза I, фаза II, фаза III и фаза IV), размер организации (малая, средняя и большая), пользовательское устройство (настольный компьютер, планшет , карманное устройство PDA, смартфон и другие), конечный пользователь (фармацевтические и биофармацевтические компании, организации по контрактным исследованиям, компании, оказывающие консалтинговые услуги, производители медицинского оборудования, больницы и научно-исследовательские институты)
|
Страны, охваченные
|
Германия, Франция, Великобритания, Италия, Испания, Нидерланды, Россия, Швейцария, Турция, Бельгия и остальные страны Европы.
|
Охваченные участники рынка
|
Oracle (США), Signant Health (США), MaxisIT (США), Parexel International Corporation (США), Merative (Индия), Dassault Systèmes (Франция), Clario (США), Mednet (США), OpenClinica LLC (США), 4G Clinical (США), Veeva Systems (США), Saama Technologies, LLC (США), Anju (США), Castor (Нидерланды), Medrio, Inc. (США), ArisGlobal (США), Advarra (США), eClinical Solutions, LLC (США), Y-Prime LLC (США), RealTime Software Solutions LLC США, Quretec (Финляндия), Research Manager (Новая Зеландия), Datatrack International (Великобритания), IQVIA Inc. (США)
|
Данные, отраженные в отчете
|
Помимо аналитических данных о рыночных сценариях, таких как рыночная стоимость, темпы роста, сегментация, географический охват и основные игроки, рыночные отчеты, подготовленные Data Bridge Market Research, также включают в себя углубленный экспертный анализ, эпидемиологию пациентов, анализ воронки продаж, анализ ценообразования и нормативную базу.
|
Анализ сегмента:
Европейский рынок электронных клинических решений разделен на шесть основных сегментов в зависимости от продукта, способа доставки, фазы клинических испытаний, размера организации, пользовательского устройства и конечного пользователя.
- На основе продукта рынок электронных клинических решений в Европе сегментирован на электронный сбор данных и управление клиническими данными, системы управления клиническими испытаниями, платформы клинической аналитики, медицинскую карту координации лечения (CCMR), рандомизацию и управление поставками для испытаний, платформы интеграции клинических данных, электронные решения для оценки клинических результатов, решения по безопасности, электронные системы основных файлов испытаний, решения по управлению нормативной информацией и другие.
- На основе способа доставки рынок электронных клинических решений в Европе сегментируется на веб-решения (по запросу), лицензированные корпоративные (локальные) решения и облачные (SAAS) решения.
- На основе фазы клинических испытаний рынок электронных клинических решений в Европе сегментирован на фазу I, фазу II, фазу III и фазу IV.
- По размеру организации рынок электронных клинических решений в Европе сегментируется на малые, средние и крупные.
- По типу пользовательского устройства рынок электронных клинических решений в Европе сегментируется на настольные компьютеры, планшеты, карманные устройства PDA, смартфоны и другие.
- По признаку конечного пользователя рынок электронных клинических решений в Европе сегментирован на фармацевтические и биофармацевтические компании, контрактные исследовательские организации, консалтинговые компании, производителей медицинского оборудования, больницы и академические научно-исследовательские институты.
Основные игроки
Компания Data Bridge Market Research выделяет следующие компании в качестве основных игроков на европейском рынке электронных клинических решений: Oracle (США), Signant Health (США), MaxisIT (США), Parexel International Corporation (США), Merative (Индия), Dassault Systèmes (Франция), Clario (США), Mednet (США), OpenClinica, LLC (США).
Развитие рынка
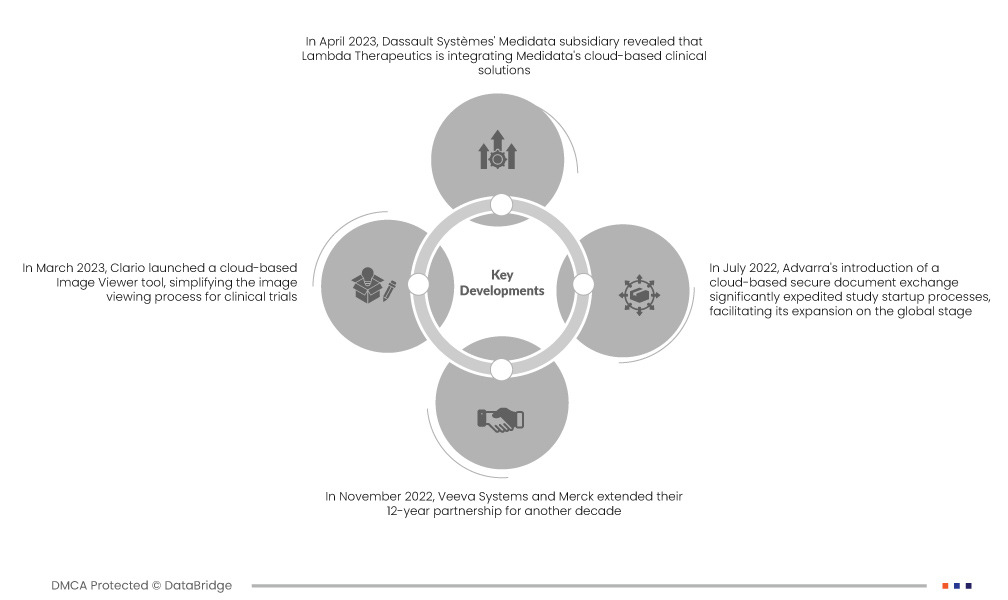
- В апреле 2023 года дочерняя компания Medidata компании Dassault Systèmes сообщила, что Lambda Therapeutics интегрирует облачные клинические решения Medidata, включая Rave EDC, Rave RTSM и Rave Imaging. Этот стратегический шаг направлен на улучшение управления данными, обеспечение качества данных и ускорение эффективности клинических испытаний, что укрепляет глобальное присутствие Medidata в отрасли
- В марте 2023 года Clario представила облачный инструмент Image Viewer, упрощающий процесс просмотра изображений для клинических испытаний . Это нововведение устраняет необходимость участия нескольких организаций в сложных процедурах передачи изображений, снижая риски, задержки и ошибки. Расширенное предложение услуг Clario повышает эффективность и надежность управления клиническими испытаниями
- В ноябре 2022 года Veeva Systems и Merck продлили свое 12-летнее партнерство еще на десятилетие. Merck будет отдавать приоритет облачным решениям Veeva в разработке отраслевого программного обеспечения, влияя на разработку продуктов и ценовую стратегию Veeva. Это сотрудничество ускоряет цифровую стратегию Merck и повышает использование продуктов и услуг Veeva.
- В июле 2022 года внедрение Advarra безопасного обмена документами на основе облака значительно ускорило процессы запуска исследований, способствуя его расширению на мировой арене. Это инновационное решение укрепило международное присутствие Advarra в отрасли
Региональный анализ
Географически отчет о рынке электронных клинических решений в Европе охватывает следующие страны: Германия, Франция, Великобритания, Италия, Испания, Нидерланды, Россия, Швейцария, Турция, Бельгия и остальные страны Европы.
Согласно анализу Data Bridge Market Research:
По оценкам, Германия станет самым быстрорастущим регионом в Европе на рынке электронных клинических решений в прогнозируемый период 2023–2030 гг.
Ожидается, что Германия будет доминировать на европейском рынке электронных клинических решений, что обусловлено растущим внедрением систем электронного сбора данных и облачных решений. Это принятие отражает растущую тенденцию к цифровизации и эффективности клинических исследований и испытаний в регионе.
Более подробную информацию об отчете о рынке электронных клинических решений в Европе можно получить здесь – https://www.databridgemarketresearch.com/reports/europe-eclinical-solutions-market