Высокая заболеваемость хроническими заболеваниями, такими как респираторные заболевания, диабет, сердечно-сосудистые заболевания и другие, как ожидается, увеличит спрос на активные фармацевтические ингредиенты (АФИ). Согласно отчету Американской диабетической ассоциации в 2018 году, 34,2 миллиона или 10,5% американцев имели диабет. Кроме того, диабет считается одной из седьмых основных причин смерти в США, поскольку к 2017 году 83 564 случая смерти были вызваны диабетом. Таким образом, это означает, что высокая распространенность хронических заболеваний, спрос на инновации активных фармацевтических ингредиентов (АФИ) растет, что приводит к росту рынка в течение прогнозируемого периода.
Доступ к полному отчету по адресу https://www.databridgemarketresearch.com/reports/global-active-pharmaceutical-ingredient-api-market
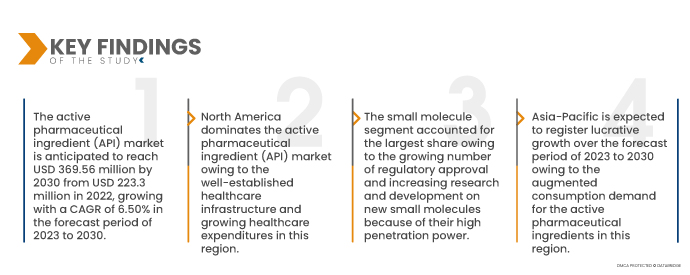
По данным Data Bridge Market Research, ожидается, что рынок активных фармацевтических ингредиентов (АФИ) достигнет 369,56 млн долларов США к 2030 году по сравнению с 223,3 млн долларов США в 2021 году, при среднегодовом темпе роста 6,50% в прогнозируемый период с 2023 по 2030 год. Увеличение инвестиций в исследования, касающиеся расширения рынка активных фармацевтических ингредиентов (АФИ), вероятно, увеличит рост мирового рынка активных фармацевтических ингредиентов (АФИ).
Ожидается , что растущее использование передовых технологий в производстве активных фармацевтических ингредиентов (АФИ) будет способствовать темпам роста рынка.
Ожидается, что глобальный рынок активных фармацевтических ингредиентов (API) значительно вырастет за счет использования передовых технологий, таких как IoT, которые повышают терапевтическую активность готовых лекарственных препаратов и улучшают удобство лекарственных препаратов. Эти инновационные технологии стимулируют рост рынка. Различные технологии используются для оптимизации активных фармацевтических ингредиентов (API) и готовых лекарственных препаратов. Например, разработка API достигла цифровой трансформации, что привело к использованию технологии Интернета вещей (IoT) и движению к автоматизации. Средства Интернета вещей (IoT) могут помочь облегчить производственный поток в производственных помещениях, поскольку они работают как автоматическое устройство мониторинга, которое может управлять складами и запасами, а также анализировать циклы разработки.
Область отчета и сегментация рынка
Отчет Метрика
|
Подробности
|
Прогнозируемый период
|
2023-2030
|
Базовый год
|
2022
|
Исторические годы
|
2021 (можно настроить на 2015-2020)
|
Количественные единицы
|
Доход в млн. долл. США, объемы в единицах, цены в долл. США
|
Охваченные сегменты
|
Молекула (малая молекула, большая молекула), тип (инновационные активные фармацевтические ингредиенты, генерические инновационные активные фармацевтические ингредиенты), тип производителя (производитель собственных API, производитель торговых API), синтез (синтетические активные фармацевтические ингредиенты и биотехнологические активные фармацевтические ингредиенты), химический синтез ( ацетаминофен , артемизинин, саксаглиптин, хлорид натрия, ибупрофен, лозартан калия, эноксапарин натрия, руфинамид, напроксен, тамоксифен и другие), тип препарата (рецептурные препараты, безрецептурные препараты), использование (клиническое, исследовательское), эффективность (активные фармацевтические ингредиенты низкой и средней эффективности, эффективные и высокоэффективные активные фармацевтические ингредиенты), терапевтическое применение (кардиология, ЦНС и неврология, онкология, ортопедия, Эндокринология, пульмонология, гастроэнтерология, нефрология, офтальмология, другие терапевтические применения)
|
Страны, охваченные
|
США, Канада и Мексика в Северной Америке, Германия, Франция, Великобритания, Нидерланды, Швейцария, Бельгия, Россия, Италия, Испания, Турция, Остальная Европа в Европе, Китай, Япония, Индия, Южная Корея, Сингапур, Малайзия, Австралия, Таиланд, Индонезия, Филиппины, Остальная часть Азиатско-Тихоокеанского региона (APAC) в Азиатско-Тихоокеанском регионе (APAC), Саудовская Аравия, ОАЭ, Южная Африка, Египет, Израиль, Остальной Ближний Восток и Африка (MEA) как часть Ближнего Востока и Африки (MEA), Бразилия, Аргентина и Остальная часть Южной Америки как часть Южной Америки
|
Охваченные участники рынка
|
Novartis AG (Швейцария), Sanofi (Франция). Pfizer Inc. (США), Johnson & Johnson Private Limited (США), Abbott (США), Teva Pharmaceutical Industries Ltd. (Израиль), Bausch Health Companies Inc. (Канада), UCB SA (Бельгия), Sunovion Pharmaceuticals Inc. (США), Jazz Pharmaceuticals, Inc. (Великобритания), AstraZeneca (Великобритания), GSK plc (Великобритания), H. Lundbeck A/S (Дания), Takeda Pharmaceutical Company Limited (Япония), Sumitomo Dainippon Pharma Co., Ltd. (Япония), Cadila Pharmaceuticals (Индия)
|
Данные, отраженные в отчете
|
Помимо аналитических данных о рыночных сценариях, таких как рыночная стоимость, темпы роста, сегментация, географический охват и основные игроки, рыночные отчеты, подготовленные Data Bridge Market Research, также включают в себя углубленный экспертный анализ, эпидемиологию пациентов, анализ воронки продаж, анализ ценообразования и нормативную базу.
|
Анализ сегмента:
Рынок активных фармацевтических ингредиентов (АФИ) сегментирован по признаку молекулы, типа, типа производителя, синтеза, химического синтеза, типа лекарственного средства, использования, эффективности и терапевтического применения.
- На основе молекулы глобальный рынок активных фармацевтических ингредиентов (API) сегментируется на малые молекулы и большие молекулы. Ожидается, что сегмент малых молекул будет доминировать на рынке с долей рынка 63,78% из-за растущего числа регулирующих одобрений и увеличения исследований и разработок новых малых молекул из-за их высокой проникающей способности.
- На основе типа глобальный рынок активных фармацевтических ингредиентов (API) сегментируется на инновационные активные фармацевтические ингредиенты и генерические активные фармацевтические ингредиенты. Ожидается, что в 2021 году сегмент инновационных активных фармацевтических ингредиентов будет доминировать на рынке с долей рынка 52,17% из-за высокой стоимости инновационных API по сравнению с генерическими API и растущего одобрения FDA для новых молекулярных объектов.
- На основе типа производителя глобальный рынок активных фармацевтических ингредиентов (API) сегментируется на производителей API-контейнеров и производителей API-контейнеров. Ожидается, что сегмент производителей API-контейнеров будет доминировать на рынке с долей рынка 61,29% из-за высоких инвестиций ключевых игроков в создание высококлассных производственных мощностей.
- По признаку синтеза мировой рынок активных фармацевтических ингредиентов (АФИ) сегментируется на синтетические активные фармацевтические ингредиенты и биотехнологические активные фармацевтические ингредиенты.
Ожидается, что сегмент синтетических активных фармацевтических ингредиентов будет доминировать на рынке активных фармацевтических ингредиентов (АФИ)
Ожидается, что сегмент синтетических активных фармацевтических ингредиентов будет доминировать на рынке с долей рынка 57,47% благодаря менее строгим нормативным требованиям и простому протоколу синтеза молекул в следующем году.
- На основе химического синтеза глобальный рынок активных фармацевтических ингредиентов (АФИ) сегментируется на ацетаминофен, артемизинин, саксаглиптин, хлорид натрия, ибупрофен, лозартан калия, эноксапарин натрия, руфинамид, напроксен, тамоксифен и другие. Ожидается, что сегмент саксаглиптина будет доминировать на рынке с долей рынка 11,76% из-за высокой цены АФИ и увеличения случаев диабета во всем мире. Кроме того, саксаглиптин является новой категорией противодиабетических средств с высокой эффективностью.
- На основе типа препарата глобальный рынок активных фармацевтических ингредиентов (АФИ) сегментируется на рецептурные препараты и безрецептурные препараты. Ожидается, что сегмент рецептурных препаратов будет доминировать на рынке с долей рынка 53,26% из-за высокой стоимости других препаратов, что увеличит себестоимость АФИ.
- На основе использования глобальный рынок активных фармацевтических ингредиентов (API) сегментируется на клинический и исследовательский. Ожидается, что клинический сегмент будет доминировать на рынке с долей рынка 66,32% из-за растущего числа заболеваний, требующих непрерывной поставки API. Кроме того, увеличение количества клинических испытаний также приписывается доминированию роста рынка.
- На основе активности мировой рынок активных фармацевтических ингредиентов (АФИ) сегментируется на активные фармацевтические ингредиенты с низкой и средней активностью и активные фармацевтические ингредиенты с высокой активностью.
Ожидается, что сегмент активных фармацевтических ингредиентов низкой и средней активности будет доминировать на рынке активных фармацевтических ингредиентов (АФИ)
Ожидается, что сегмент активных фармацевтических ингредиентов с низкой и средней активностью будет доминировать на рынке с долей рынка 56,52% ввиду их меньшей опасности и меньшей токсичности.
- На основе терапевтического применения глобальный рынок активных фармацевтических ингредиентов (API) сегментируется на кардиологию, ЦНС и неврологию, онкологию, ортопедию, эндокринологию, пульмонологию, гастроэнтерологию, нефрологию, офтальмологию и другие терапевтические применения. Ожидается, что сегмент больничной аптеки будет доминировать на рынке с долей рынка 19,05% из-за растущего числа сердечных заболеваний, таких как эмболия, сердечный приступ и другие.
Основные игроки
Data Bridge Market Research признает следующие компании основными игроками на рынке активных фармацевтических ингредиентов (АФИ): Novartis AG (Швейцария), Sanofi (Франция). Pfizer Inc. (США), Johnson & Johnson Private Limited (США), Abbott (США), Teva Pharmaceutical Industries Ltd. (Израиль), Bausch Health Companies Inc. (Канада), UCB SA (Бельгия), Sunovion Pharmaceuticals Inc. (США), Jazz Pharmaceuticals, Inc. (Великобритания).
Развитие рынка

- В 2020 году Bright Path Laboratories и Quartic.ai подписали контракт на производство активных фармацевтических ингредиентов (API) с использованием технологии искусственного интеллекта. Искусственный интеллект (AI) является одной из инновационных и передовых платформ для открытия лекарств. Был подписан контракт, который включает Quartic.ai Технология ИИ может резко увеличить скорость проектирования, утверждения и валидации активных фармацевтических ингредиентов (API) на основе ИИ. Bright Path Laboratories будет использовать свою технологию Flow Spinning Tube-in-Tube, которая может формировать большое количество API и химикатов, которые будут поставлять продукт высокой степени чистоты. Сочетание этих технологий увеличит их продуктовый портфель с точки зрения инновационных продуктов.
- В 2020 году CordenPharma International объявила о завершении строительства своей высокоэффективной лаборатории API в Колорадо. Эта новая лаборатория будет заниматься коммерциализацией и производством API, а целью компании было удовлетворение спроса клиентов. Это помогло компании расширить свой продуктовый портфель и увеличить доходы в предстоящий период.
Региональный анализ
Географически в отчете о рынке активных фармацевтических ингредиентов (АФИ) рассматриваются следующие страны: США, Канада и Мексика в Северной Америке, Германия, Франция, Великобритания, Нидерланды, Швейцария, Бельгия, Россия, Италия, Испания, Турция, остальные страны Европы в Европе, Китай, Япония, Индия, Южная Корея, Сингапур, Малайзия, Австралия, Таиланд, Индонезия, Филиппины, остальные страны Азиатско-Тихоокеанского региона (APAC) в Азиатско-Тихоокеанском регионе (APAC), Саудовская Аравия, ОАЭ, Южная Африка, Египет, Израиль, остальные страны Ближнего Востока и Африки (MEA) как часть Ближнего Востока и Африки (MEA), Бразилия, Аргентина и остальные страны Южной Америки как часть Южной Америки.
Согласно анализу Data Bridge Market Research:
Северная Америка будет доминирующим регионом на рынке активных фармацевтических ингредиентов (АФИ) в прогнозируемый период с 2023 по 2030 год.
Северная Америка доминирует на рынке активных фармацевтических ингредиентов (API) благодаря хорошо налаженной инфраструктуре здравоохранения и растущим расходам на здравоохранение в этом регионе. Кроме того, присутствие основных ключевых игроков и более широкое внедрение синтетических активных фармацевтических ингредиентов (API) еще больше ускорит рост рынка в этом регионе.
По оценкам, Азиатско-Тихоокеанский регион станет самым быстрорастущим регионом на рынке активных фармацевтических ингредиентов (АФИ) в прогнозируемый период с 2023 по 2030 год.
Ожидается, что Азиатско-Тихоокеанский регион будет расти в прогнозируемый период с 2023 по 2030 год из-за возросшего спроса на активные фармацевтические ингредиенты в этом регионе. Кроме того, растущее развитие инфраструктуры здравоохранения и растущие правительственные инициативы являются некоторыми из других основных факторов, которые будут и дальше стимулировать рост рынка этого региона.
Для получения более подробной информации об отчете по рынку активных фармацевтических ингредиентов (API) нажмите здесь – https://www.databridgemarketresearch.com/reports/global-active-pharmaceutical-ingredient-api-market










