The market for adalimumab is expected to expand quickly over the next few years. The drug adalimumab, first authorized in the United States, is now accessible in more than 60 nations. Few companies are competing against one another on price in its congested global market. The majority of the major players are presently focusing their efforts on the creation of adalimumab biosimilars for the management of psoriasis and rheumatoid arthritis. Clinical trials examining the security and effectiveness of adalimumab biosimilars in the treatment of medical conditions.
Access Full Report @ https://www.databridgemarketresearch.com/reports/global-adalimumab-market
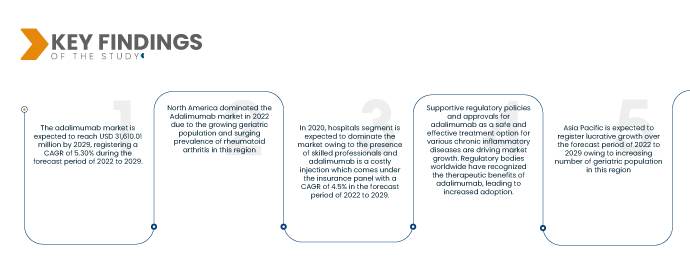
Data Bridge Market Research analyses that, the Adalimumab Market was valued at USD 20,912.1 million in 2021 and is expected to reach USD 31,610.01 million by 2029, registering a CAGR of 5.30% during the forecast period of 2022 to 2029. Adalimumab is primarily prescribed for the treatment of autoimmune diseases such as rheumatoid arthritis, psoriasis, Crohn's disease, ulcerative colitis, and ankylosing spondylitis. The rising incidence and prevalence of these conditions worldwide have contributed to the growth of the adalimumab market.
Technological advancements and improved formulations is expected to drive the market's growth rate
Continuous advancements in drug delivery systems and formulation technologies for adalimumab have led to improved treatment outcomes and patient experiences. Innovations like autoinjectors and prefilled syringes offer convenient and user-friendly options for administering adalimumab, reducing the need for healthcare visits and enhancing patient compliance. These advancements enhance the effectiveness, convenience, and overall patient satisfaction, contributing to the growth of the adalimumab market.
Report Scope and Market Segmentation
|
Report Metric
|
Details
|
|
Forecast Period
|
2022 to 2029
|
|
Base Year
|
2021
|
|
Historic Years
|
2020 (Customizable to 2014-2019)
|
|
Quantitative Units
|
Revenue in USD Million, Volumes in Units, Pricing in USD
|
|
Segments Covered
|
Drug Class (Antirheumatics, TNF Alfa Inhibitors, Others), Indication (Rheumatoid Arthritis, Ankylosing Spondylitis, Chronic Plaque Psoriasis, Crohn's Disease, Ulcerative Colitis, Psoriatic Arthritis, Juvenile Idiopathic Arthritis, Hidradenitis Suppurativa, Non-Infectious Intermediate, Others), Type (Biologics, Biosimilars), Dosage Strength (40mg/0.4mlg, 80mg/0.8mlg, 20mg/0.2mlg, 10mg/0.1mlg, Others), Drug Type (Branded, Generics), Route of Administration (Oral, Parenteral, Others), Age Group (Pediatric, Adult, Geriatric), Dosage Form (Tablet, Injection, Solution, Others), End-Users (Hospitals, Specialty Clinics, Homecare, Others), Distribution Channel (Hospital Pharmacy, Retail Pharmacy, Online Pharmacy, Others)
|
|
Countries Covered
|
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America
|
|
Market Players Covered
|
F. Hoffmann-La Roche Ltd. (Switzerland), Mylan N.V. (U.S.), Teva Pharmaceutical Industries Ltd.(Israel), Sanofi (France), Pfizer Inc. (U.S.), GlaxoSmithKline plc (U.K.), Novartis AG (Switzerland), Zydus Cadila (India), Biogen (U.S.), Fresenius Kabi AG (Germany), Boehringer Ingelheim International GmbH. (Germany), Amgen Inc. (U.S.), AbbVie Inc. (U.S.), Abbott (U.S.), CELLTRION INC. (South Korea), Samsung Bioepis (South Korea), Coherus BioSciences (U.S.), Innovent Biologics, Inc. (China), Hetero Biopharma Ltd. (India), Reliance Life Sciences (India)
|
|
Data Points Covered in the Report
|
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
|
Segment Analysis:
The adalimumab market is segmented on the basis of drug class, type, indication, dosage form, dosage strength, drug type, route of administration, age group, end-users and distribution channel.
- On the basis of drug class, the global adalimumab market is segmented into Antirheumatics, TNF Alfa Inhibitors, Others. TNF Alpha inhibitors dominate the segment because these medications specifically target and inhibit the activity of tumor necrosis factor-alpha, which plays a key role in inflammation.
- On the basis of indication, the global adalimumab market is segmented into rheumatoid arthritis, ankylosing spondylitis, chronic plaque psoriasis, crohn's disease, ulcerative colitis, psoriatic arthritis, juvenile idiopathic arthritis, hidradenitis suppurativa, non-infectious intermediate, others. rheumatoid arthritis segment is expected to dominate the market with a 4.9% in the forecast period of 2022 to 2029 due to it is a very common autoimmune systemic inflammatory disease affecting approximately 1% of the worldwide population.
- On the basis of type, the global adalimumab market is segmented into biologics and biosimilars. Biologics segment is expected to dominate the market with a CAGR of -7.1% in the forecast period of 2022 to 2029 due to less number of biosimilars present worldwide.
In 2022, biologics segment is expected to dominate the type segment of the global adalimumab market.
In 2022 biologics segment is expected to dominate the market owing to the less number of biosimilars present worldwide with a CAGR of 49.2% during the forecast period of 2022 to 2029.
- On the basis of dosage strength, global adalimumab market is segmented into 40mg/0.4mlg, 80mg/0.8mlg, 20mg/0.4mlg, 10mg/0.1mlg, and others. 40mg/0.4mlg segment is expected to dominate the market with a 5.5% in the forecast period of 2022 to 2029 due to the majority of the population or patients suffering from RA or other inflammatory diseases using 40mg dose and it’s the most useable dose for the treatment.
- On the basis of drug type, global adalimumab market is segmented into branded, generics. Branded segment is expected to dominate the market with a CAGR of -7.1% in the forecast period of 2022 to 2029 due to their extensive clinical trials, marketing efforts, and promotion by pharmaceutical companies
- On the basis of route of administration, global adalimumab market is segmented into parenteral, and oral. Parenteral segment is further sub- segment into subcutaneous, intramuscular. parenteral segment is expected to dominate the market with a CAGR of 4.8% in the forecast period of 2022 to 2029 due to a human monoclonal antibody can only be given through SC route for the long term effect.
- On the basis of age group, global adalimumab market is segmented into pediatric, adult, geriatric. Adults segment is expected to dominate the market with a CAGR of 4.6% in the forecast period of 2022 to 2029 due to majority of population suffering from inflammatory diseases are adult population and majority of clinical data supporting the adult population data.
- On the basis of dosage form, the global adalimumab market is segmented into tablet, injection, solution, and others. Tablets is expected to dominate the market due to their convenience, ease of administration, precise dosing, stability, and wide availability.
- On the basis of end-users, global adalimumab market is segmented into hospitals, specialty clinics, home healthcare, others. Hospitals segment is expected to dominate the market with a CAGR of 4.5% in the forecast period of 2022 to 2029 due to the presence of skilled professionals and adalimumab is a costly injection which comes under the insurance panel.
In 2022 hospitals segment is expected to dominate the end-users segment of the adalimumab market
In 2022 hospitals segment is expected to dominate the market owing to the presence of skilled professionals and adalimumab is a costly injection which comes under the insurance panel with a CAGR of 4.5% in the forecast period of 2022 to 2029.
- On the basis of distribution channel, global adalimumab market is segmented into hospital pharmacies, retail pharmacies, online pharmacies, direct tenders, others. Hospital pharmacies segment is expected to dominate the market with a CAGR of 4.9% in the forecast period of 2022 to 2029 due to the presence of experienced professional and adalimumab is only prescribed by the doctors, so one can only buy from the hospital pharmacies at a reasonable price.
Major Players
Data Bridge Market Research recognizes the following companies as the major adalimumab market players in Adalimumab market are F. Hoffmann-La Roche Ltd. (Switzerland), Mylan N.V. (U.S.), Teva Pharmaceutical Industries Ltd.(Israel), Sanofi (France), Pfizer Inc. (U.S.), GlaxoSmithKline plc (U.K.), Novartis AG (Switzerland), Zydus Cadila (India), Biogen (U.S.), Fresenius Kabi AG (Germany)
Market Development
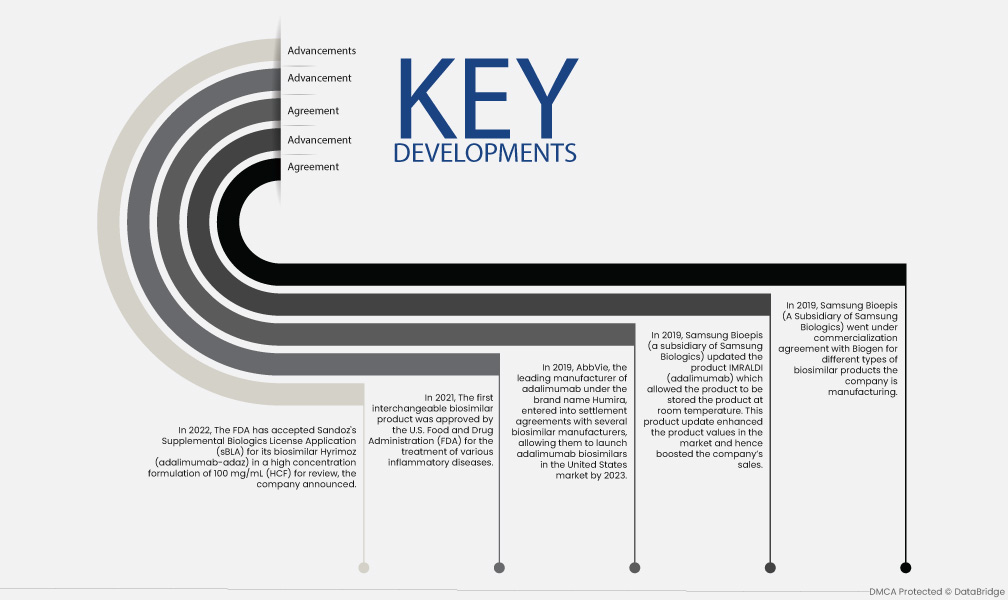
- In 2022, The FDA has accepted Sandoz's Supplemental Biologics License Application (sBLA) for its biosimilar Hyrimoz (adalimumab-adaz) in a high concentration formulation of 100 mg/mL (HCF) for review, the company announced.
- In 2021, The first interchangeable biosimilar product was approved by the U.S. Food and Drug Administration (FDA) for the treatment of various inflammatory diseases.
- In 2019, AbbVie, the leading manufacturer of adalimumab under the brand name Humira, entered into settlement agreements with several biosimilar manufacturers, allowing them to launch adalimumab biosimilars in the United States market by 2023.
- In 2019, Samsung Bioepis (a subsidiary of Samsung Biologics) updated the product IMRALDI (adalimumab) which allowed the product to be stored the product at room temperature. This product update enhanced the product values in the market and hence boosted the company’s sales.
- In 2019, Samsung Bioepis (A Subsidiary of Samsung Biologics) went under a commercialization agreement with Biogen for differenf biosimilar products the companmanufacturesng.
Regional Analysis
Geographically, the countries covered in the adalimumab market report are U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America
As per Data Bridge Market Research analysis:
North America is the dominant region in adalimumab market during the forecast period 2022 to 2029
North America holds a prominent position in the adalimumab market due to several factors. The region's growing geriatric population, coupled with the increasing prevalence of rheumatoid arthritis and other chronic inflammatory diseases, drives the demand for adalimumab. Furthermore, the region's high healthcare expenditure and strong healthcare infrastructure contribute to the market's dominance. The continuous investment in advanced healthcare technologies and therapies, along with favorable reimbursement policies, supports the growth rate of the adalimumab market in North America.
Asia-Pacific is estimated to be the fastest growing region in adalimumab market the forecast period 2022 to 2029
The Asia-Pacific region is projected to experience significant growth in the adalimumab market from 2022 to 2029. Factors driving this growth include the increasing focus on developing generic and biosimilar products, as well as the growing geriatric population in the region. Additionally, the development of healthcare infrastructure, including hospitals and clinics, will further support the market's expansion by improving access to adalimumab treatments. These factors collectively contribute to the anticipated growth rate of the market in the Asia-Pacific region.
For more detailed information about the adalimumab market report, click here – https://www.databridgemarketresearch.com/reports/global-adalimumab-market