The adalimumab market has witnessed significant innovations and advancements, primarily in biosimilar versions, which offer cost-effective alternatives to the brand-name drug, expanding patient access. Ongoing research focuses on personalized dosing regimens and novel drug delivery mechanisms, enhancing treatment precision and patient convenience. These developments reflect a commitment to improving the efficacy, affordability, and accessibility of adalimumab therapy for various chronic inflammatory conditions, such as rheumatoid arthritis, Crohn's disease, and psoriasis, ultimately benefiting a broader spectrum of patients.
Access Full Report @ https://www.databridgemarketresearch.com/reports/europe-adalimumab-market
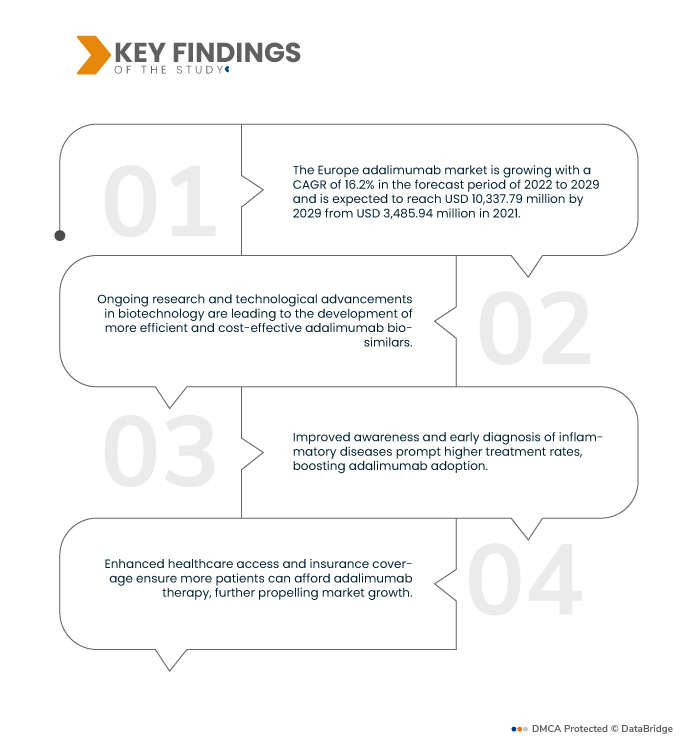
Data Bridge Market Research analyses that the Europe Adalimumab Market is growing with a CAGR of 16.2% in the forecast period of 2022 to 2029 and is expected to reach USD 10,337.79 million by 2029 from USD 3,485.94 million in 2021. The increasing prevalence of rheumatoid arthritis, Crohn's disease, and psoriasis necessitate effective management, driving demand for adalimumab due to its proven efficacy in treating these chronic inflammatory conditions.
Key Findings of the Study
Expanding aging population is expected to drive the market's growth rate
As the population ages, individuals are more susceptible to chronic inflammatory conditions such as rheumatoid arthritis, Crohn's disease, and psoriasis. Adalimumab's efficacy in managing these ailments becomes crucial for improving the quality of life among the elderly. Consequently, the growing aging demographic fuels the demand for adalimumab, contributing to market expansion as healthcare providers and patients increasingly rely on this treatment to address the specific needs of this vulnerable population.
Report Scope and Market Segmentation
|
Report Metric
|
Details
|
|
Forecast Period
|
2022 to 2029
|
|
Base Year
|
2021
|
|
Historic Years
|
2020 (Customizable to 2014-2019)
|
|
Quantitative Units
|
Revenue in USD Million, Volumes in Units, Pricing in USD
|
|
Segments Covered
|
Indication (Rheumatoid Arthritis, Juvenile Idiopathic Arthritis, Psoriatic Arthritis, Ankylosing Spondylitis, Crohn's Disease, Hidradenitis Suppurativa, Ulcerative Colitis, Chronic Plaque Psoriasis, Non-Infectious Intermediate, and Others), Type (Biologics and Biosimilars), Dosage Strength (20MG/0.4MLG, 40MG/0.8MLG and Others), Drug Type (Humira, Amgevita, Imraldi, Hyrimoz, Yuflyma, Hulio, Idacio), Population Type (Children and Adults), End User (Hospitals, Specialty Clinics, Home Healthcare, and Others), Distribution Channel (Hospital Pharmacies, Retail Pharmacies, Online Pharmacies, and Others)
|
|
Countries Covered
|
Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe.
|
|
Market Players Covered
|
AbbVie Inc. (U.S.), Sandoz International GmbH (Germany), Amgen Inc. (U.S.), Mylan N.V. (A subsidiary of Viatris) (U.S.), Biogen (U.S.), Celltrion Healthcare Co., Ltd. (South Korea), Fresenius Kabi SwissBioSim GmbH (Germany), Alvotech (Iceland), Biocad (Russia), Coherus BioSciences (U.S.), Shanghai Henlius Biotech, Inc. (China), Synermore Biologics (China), Prestige BioPharma Ltd. (Singapore), Janssen Global Services, LLC (U.S.)
|
|
Data Points Covered in the Report
|
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
|
Segment Analysis:
The Europe adalimumab market is segmented into indication, type, dosage strength, drug type, population type, end user, and distribution channel.
- On the basis of indication, Europe adalimumab market is segmented into rheumatoid arthritis, juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn’s disease, hidradenitis suppurativa, ulcerative colitis, chronic plaque psoriasis, non-infectious intermediate, and others.
- On the basis of type, Europe adalimumab market is segmented into biologics and biosimilars.
- On the basis of dosage strength, Europe adalimumab market is segmented into 20MG/0.4MLG, 40MG/0.8MLG, and others.
- On the basis of drug type, Europe adalimumab market is segmented into Humira, Amgevita, Imraldi, Hyrimoz, Yuflyma, Hulio and Idacio.
- On the basis of population type, Europe adalimumab market is segmented into children and adults.
- On the basis of end user, Europe adalimumab market is segmented into hospital, specialty clinics, home healthcare, and others.
- On the basis of distribution channel, Europe adalimumab market is segmented into hospital pharmacies, retail pharmacies, online pharmacies, and others.
Major Players
Data Bridge Market Research recognizes the following companies as the Europe adalimumab market players in Europe adalimumab market are AbbVie Inc. (U.S.), Sandoz International GmbH (Germany), Amgen Inc. (U.S.), Mylan N.V. (A subsidiary of Viatris) (U.S.), Biogen (U.S.), Celltrion Healthcare Co., Ltd. (South Korea), Fresenius Kabi SwissBioSim GmbH (Germany).
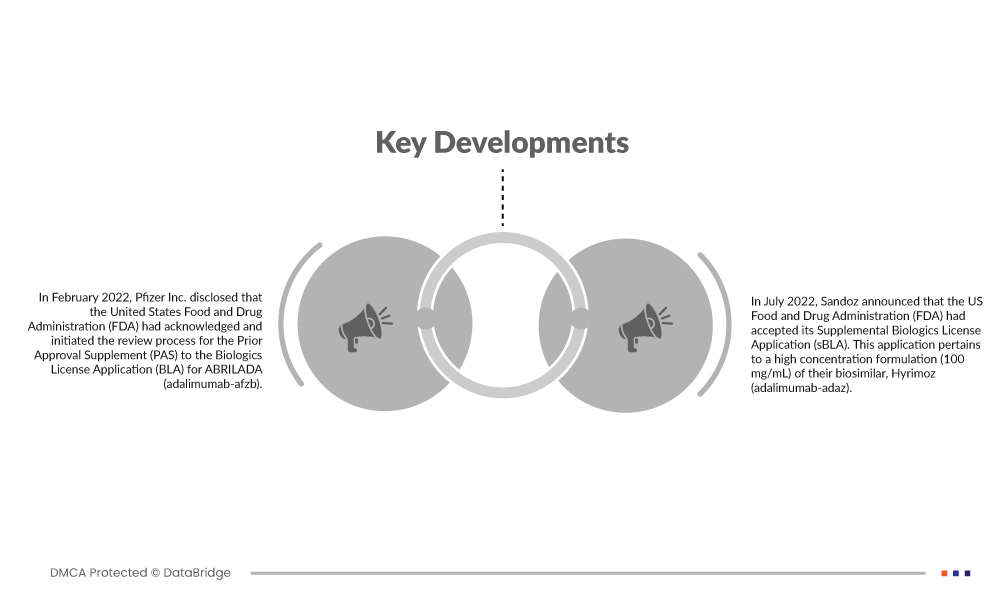
Market Developments
- In February 2022, Pfizer Inc. disclosed that the United States Food and Drug Administration (FDA) had acknowledged and initiated the review process for the Prior Approval Supplement (PAS) to the Biologics License Application (BLA) for ABRILADA (adalimumab-afzb). This review aims to determine if ABRILADA can be designated as an interchangeable biosimilar to Humira (adalimumab), a widely used medication for various chronic inflammatory conditions.
- In July 2022, Sandoz announced that the US Food and Drug Administration (FDA) had accepted its Supplemental Biologics License Application (sBLA). This application pertains to a high concentration formulation (100 mg/mL) of their biosimilar, Hyrimoz (adalimumab-adaz). It encompasses the same indications as the reference medicine Humira (adalimumab) that are not protected by orphan exclusivity. These indications include conditions such as rheumatoid arthritis, juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn's disease, ulcerative colitis, and plaque psoriasis.
Regional Analysis
Geographically, the countries covered in the Europe adalimumab market report are Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe.
As per Data Bridge Market Research analysis:
Germany is the dominant region in the Europe adalimumab market during the forecast period 2022-2029
Germany dominates the adalimumab market, both in terms of market share and revenue, and this trend is expected to persist throughout the forecast period. The market's success is attributed to the increasing prevalence of chronic diseases and the significant investments in research and development related to drug development in the German region. These factors collectively contribute to Germany's ongoing growth and leadership in the adalimumab market.
For more detailed information about the Europe adalimumab market report, click here – https://www.databridgemarketresearch.com/reports/europe-adalimumab-market