The availability of numerous cervical cancer diagnostic products and brands, as well as the rise in market participants, are both contributing to the growth of the global market for cervical cancer diagnostics in the anticipated year. Additionally, market participants are working on cutting-edge cervical cancer diagnostics. The market is being given opportunities by numerous government and private partnerships, rising Research and development activities, and strategic moves by market participants. False cervical cancer screening test results are anticipated to be a major barrier to market expansion, though.
Access Full Report @ https://www.databridgemarketresearch.com/reports/global-cervical-cancer-diagnostic-market
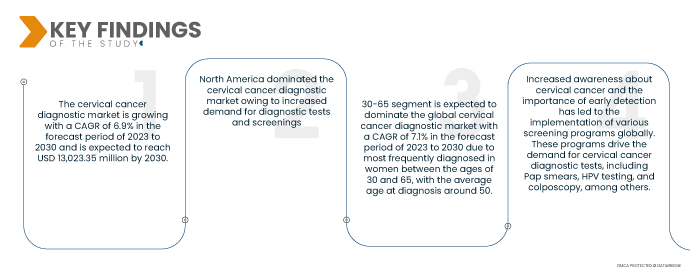
Data Bridge Market Research analyses that the Cervical Cancer Diagnostic Market is growing with a CAGR of 6.9% in the forecast period of 2023 to 2030 and is expected to reach USD 13,023.35 million by 2030. The rising incidence of cervical cancer globally is a significant driver for the diagnostic market. Cervical cancer is one of the most common cancers in women, and the need for early detection and accurate diagnosis fuels the demand for diagnostic tools and tests.
Key Findings of the Study
Increasing adoption of HPV vaccination is expected to drive the market's growth rate
The increasing adoption of HPV vaccination as a preventive measure against cervical cancer has created a need for diagnostic tests in the global market. These tests are essential to monitor the effectiveness of the vaccine and identify breakthrough infections in vaccinated individuals. Providing insights into the presence of HPV infections and assessing vaccine efficacy, diagnostic tests play a crucial role in guiding treatment decisions and ensuring the success of HPV vaccination programs, thus driving the demand in the market.
Report Scope and Market Segmentation
|
Report Metric
|
Details
|
|
Forecast Period
|
2023 to 2030
|
|
Base Year
|
2022
|
|
Historic Years
|
2021 (Customizable to 2015-2020)
|
|
Quantitative Units
|
Revenue in USD Million, Volumes in Units, Pricing in USD
|
|
Segments Covered
|
Product Type (Imaging Test, Screening Test, Visual Examination, Cervical Biopsies, and Other Procedures), Age Group (Below 21, 21-29, 30-65, 65 and Above), Stages (Stage I, Stage II, Stage III, Stage IV), End Users (Hospitals, Diagnostic Laboratories, Specialty Clinics, Community Health Centers, Cancer Research Organization, Cancer and Radiation Therapy Centers), Distribution Channel (Direct Tender, Retail Sales, Online Sales)
|
|
Countries Covered
|
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
|
|
Market Players Covered
|
Siemens Healthcare GmbH (Germany), BD (Becton, Dickinson and Company) (U.S.), F. Hoffmann-La Roche Ltd (Switzerland), Abbott (U.S.),Hologic, Inc. (U.S.),Quest Diagnostics Incorporated (U.S.),Bio-Rad Laboratories, Inc. (U.S.),QIAGEN (Netherlands), The Cooper Companies Inc. (U.S.),Seegene Inc. (South Korea), Sysmex Corporation (Japan), MobileODT (Israel), Zilico (U.K.)Jiangsu Mole Bioscience Co. Ltd. (China), Guided Therapeutics, Inc. (U.S.),GenomeMe Lab Inc. (South Korea), Arbor Vita Corporation (U.S.), LCM GENECT Srl (Italy), among others
|
|
Data Points Covered in the Report
|
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
|
Segment Analysis:
The cervical cancer diagnostic market is segmented on the basis of product type, wound type, wound class, end user, and distribution channel.
- On the basis of product type, the global cervical cancer diagnostic market is segmented into imaging test, screening test, visual examination, cervical biopsies, and other procedures. In 2023, the screening test segment is expected to dominate the global cervical cancer diagnostic market with a CAGR of 7.2% in the forecast period of 2023 to 2030 due to prevent cancer and help find cancer at an early stage and thus has more demand in the market.
In 2023, the screening test segment is expected to dominate the product type segment of the global cervical cancer diagnostic market
In 2023, the screening test segment is expected to dominate the global cervical cancer diagnostic market owing to typically performed on a large scale to detect early signs of diseases and enable early intervention, which can improve treatment outcomes with a CAGR of 7.2% in the forecast period of 2023 to 2030
- On the basis of age group, the global cervical cancer diagnostic market is segmented into below 21, 21-29, 30-65, 65 and above. In 2023, the 30-65 segment is expected to dominate the global cervical cancer diagnostic market with a CAGR of 7.1% in the forecast period of 2023 to 2030 due to continued recognized in women between the ages of 30 and 65, with the average age at diagnosis around 50.
- On the basis of stages, the global cervical cancer diagnostic market is segmented into stage I, stage II, stage III, and stage IV. In 2023, the stage I segment is expected to dominate the global cervical cancer diagnostic market with a CAGR of 7.7% in the forecast period of 2023 to 2030 due to stage I cervical cancer patients are more prevalent as symptoms develop at an early stage.
- On the basis of end user, the global cervical cancer diagnostic market is segmented into hospitals, diagnostic laboratories, specialty clinics, community health centers, cancer research organization, and cancer and radiation therapy centers. In 2023, the cancer and radiation therapy centers segment is expected to dominate the global cervical cancer diagnostic market with a CAGR of 8.4% in the forecast period of 2023 to 2030 due to high demand being the most convenient and preferred by the people.
In 2023, the cancer and radiation therapy centers segment is expected to dominate the end user segment of the global cervical cancer diagnostic market
In 2023, the cancer and radiation therapy centers segment is expected to dominate the global cervical cancer diagnostic market owing to a dedicated hub for both patients and medical professionals involved in cancer treatment and radiation therapy with a CAGR of 8.4% in the forecast period of 2023 to 2030
- On the basis of distribution channel, the global cervical cancer diagnostic market is segmented into direct tender, retail sales, and online sales. In 2023, the direct tender segment is expected to dominate the global cervical cancer diagnostic market with a CAGR of 7.3% in the forecast period of 2023 to 2030 due to leads to cheaper costs, saves time, and has easy accessibility, thus having high demand.
Major Players
Data Bridge Market Research recognizes the following companies as the major cervical cancer Diagnostic market players in cervical cancer diagnostic market are Siemens Healthcare GmbH (Germany), BD (Becton, Dickinson and Company) (U.S.), F. Hoffmann-La Roche Ltd (Switzerland), Abbott (U.S.),Hologic, Inc. (U.S.),Quest Diagnostics Incorporated (U.S.),Bio-Rad Laboratories, Inc. (U.S.),QIAGEN (Netherlands), The Cooper Companies Inc. (U.S.),Seegene Inc. (South Korea)
Market Development
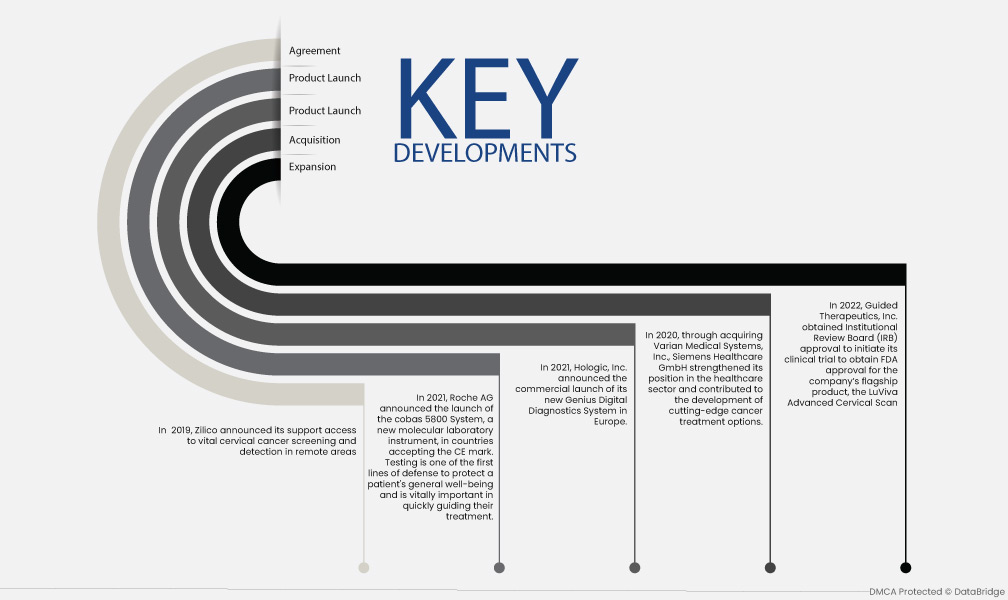
- In 2022, Guided Therapeutics, Inc. obtained Institutional Review Board (IRB) approval to initiate its clinical trial to obtain FDA approval for the company’s flagship product, the LuViva Advanced Cervical Scan
- In 2020, through acquiring Varian Medical Systems, Inc., Siemens Healthcare GmbH strengthened its position in the healthcare sector and contributed to the development of cutting-edge cancer treatment options.
- In 2021, Hologic, Inc. announced the commercial launch of its new Genius Digital Diagnostics System in Europe.
- In 2021, Roche AG announced the launch of the cobas 5800 System, a new molecular laboratory instrument, in countries accepting the CE mark. Testing is one of the first lines of defense to protect a patient's general well-being and is vitally important in quickly guiding their treatment.
- In 2019, Zilico announced its support access to vital cervical cancer screening and detection in remote areas
Regional Analysis
Geographically, the countries covered in the cervical cancer diagnostic market report are U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
As per Data Bridge Market Research analysis:
North America is expected to dominate the region in the cervical cancer diagnostic market during the forecast period 2023-2030
The U.S. is expected to dominate the cervical cancer diagnostic market in the North American region due to several factors. Firstly, the U.S. has a higher level of awareness among the population regarding cervical cancer and the importance of early diagnosis. This leads to increased demand for diagnostic tests and screenings. Secondly, the U.S. has a well-established healthcare infrastructure and access to advanced diagnostic technologies, enabling efficient and widespread cervical cancer diagnostics. These factors position the U.S. as a dominant player in the market within the North American region.
For more detailed information about the cervical cancer diagnostic market report, click here – https://www.databridgemarketresearch.com/reports/global-cervical-cancer-diagnostic-market