The circulating tumor cells (CTC) liquid biopsy market encompasses diverse technologies, including CTC detection and enrichment methods, molecular (RNA)-based technologies, and functional in vitro cell invasion assays. Other innovations include ex vivo positive selection, xenotransplantation models, microchips, single spiral microchannels, and adverse selection and immunocytochemical technologies. These advanced techniques cater to the evolving landscape of cancer diagnosis, offering a comprehensive range of tools for detecting, isolating, and analyzing circulating tumor cells, reflecting the market's commitment to precision medicine and early cancer detection.
Access Full Report @ https://www.databridgemarketresearch.com/reports/global-circulating-tumor-cells-ctc-liquid-biopsy-market
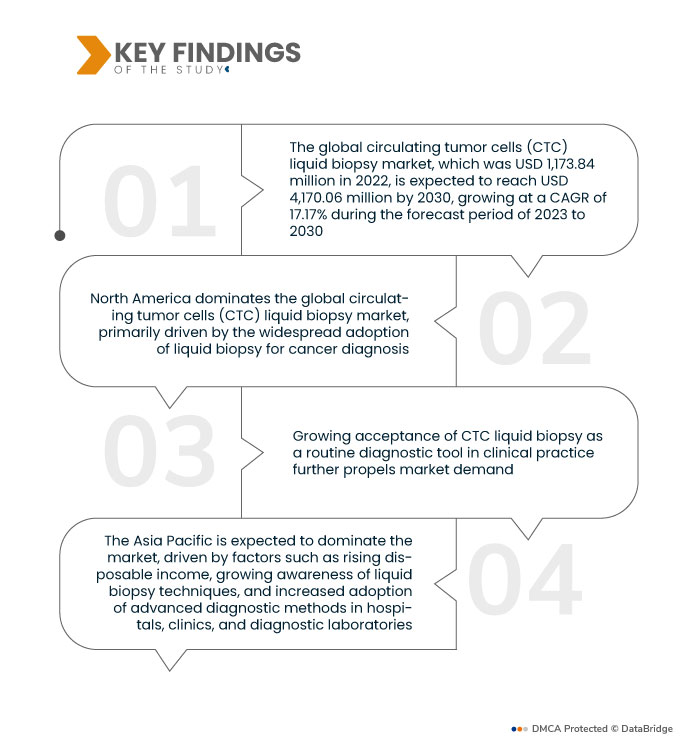
Data Bridge Market Research analyses that the Global Circulating Tumor Cells (CTC) Liquid Biopsy Market, which was USD 1,173.84 million in 2022, is expected to reach USD 4,170.06 million by 2030, growing at a CAGR of 17.17% during the forecast period of 2023 to 2030. The proliferation of diagnostic laboratories equipped with advanced technologies drives the circulating tumor cells (CTC) liquid biopsy market by enhancing accessibility to CTC liquid biopsy services, facilitating broader adoption and utilization of these advanced diagnostic techniques in clinical practice.
Key Findings of the Study
Increasing cancer cases is expected to drive the market's growth rate
The escalating global cancer prevalence is pivotal in the circulating tumor cells (CTC) liquid biopsy market. The demand for non-invasive and early detection methods is intensified as patients and healthcare professionals seek more effective and less invasive diagnostic approaches. This surge in demand significantly boosts the adoption of CTC liquid biopsy, positioning it as a preferred choice for detecting and monitoring cancer, thereby driving the market's growth trajectory.
Report Scope and Market Segmentation
|
Report Metric
|
Details
|
|
Forecast Period
|
2023 to 2030
|
|
Base Year
|
2022
|
|
Historic Years
|
2021 (Customizable to 2015-2020)
|
|
Quantitative Units
|
Revenue in USD Million, Volumes in Units, Pricing in USD
|
|
Segments Covered
|
Technology (CTC Detection Methods, CTC Enrichment Methods, Ex Vivo Positive Selection, Molecular (RNA)-Based Technologies, Functional In Vitro Cell Invasion Assay, Xenotransplantation Models, Microchips, Single Spiral Microchannel, Negative Selection and Immunocytochemical Technologies), Application (Cancer Application, Non-Cancer Applications, Non-Invasive Prenatal Testing (NIPT), Organ Transplantation, Infectious Disease Testing), Clinical Application (Early Cancer Screening, Diagnosis, Therapy Selection, Treatment Monitoring, Prognosis and Recurrence Monitoring), Sample Type, (Blood, Urine, Saliva, Cerebrospinal Fluid, Others), Product (Kits and Reagents, Blood Collection Tubes, Devices or System), Specimen (Blood, Bone Marrow, Other Body Fluids), End User (Research and Academic Institutes, Reference Laboratories and Hospitals and Physician Laboratories)
|
|
Countries Covered
|
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
|
|
Market Players Covered
|
QIAGEN (Germany), Bio-Techne (U.S.), Precision Medical Group, LLC. (U.S.), Aviva Systems Biological Corporation (U.S.), Biocept, Inc. (U.S.), Fluxion Biosciences, Inc. (U.S.), Greiner Bio-One International GmbH (Austria) , Ikonisys (U.S.), Miltenyi Biotec (Germany), IVDiagnostics (U.S.), BioFluidica (U.S.), Canopus Medical (Sweden), Biolidics Limited (Singapore), Creativ MicroTech, Inc. (U.S.), LungLife AI, Inc. (U.S.), Epic Sciences (U.S.), Rarecells Diagnostics (France), ScreenCell (France), Menarini Silicon Biosystems (U.S.).
|
|
Data Points Covered in the Report
|
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
|
Segment Analysis:
The global circulating tumor cells (CTC) liquid biopsy market is segmented on the basis of technology, application, clinical application, sample type, product, specimen, and end user.
- On the basis of technology, the global circulating tumor cells (CTC) liquid biopsy market is segmented into CTC detection methods, CTC enrichment methods, ex vivo positive selection, molecular (RNA)-based technologies, functional in vitro cell invasion assay, xenotransplantation models, microchips, single spiral microchannel, negative selection and immunocytochemical technologies
- On the basis of application, the global circulating tumor cells (CTC) liquid biopsy market is segmented into cancer application, non-cancer applications, non-invasive prenatal testing (NIPT), organ transplantation, infectious disease testing
- On the basis of clinical application, the global circulating tumor cells (CTC) liquid biopsy market is segmented into early cancer screening, diagnosis, therapy selection, treatment monitoring, prognosis and recurrence monitoring
- On the basis of sample type, the global circulating tumor cells (CTC) liquid biopsy market is segmented into blood, urine, saliva, cerebrospinal fluid, others
- On the basis of product, the global circulating tumor cells (CTC) liquid biopsy market is segmented into kits and reagents, blood collection tubes, devices, or system
- On the basis of specimen, the global circulating tumor cells (CTC) liquid biopsy market is segmented into blood, bone marrow, and other body fluids
- On the basis of end user, the global circulating tumor cells (CTC) liquid biopsy market is segmented into research and academic institutes, reference laboratories hospitals and physician laboratories
Major Players
Data Bridge Market Research recognizes the following companies as the global circulating tumor cells (CTC) liquid biopsy market players in global circulating tumor cells (CTC) liquid biopsy market are QIAGEN (Germany), Bio-Techne (U.S.), Precision Medical Group, LLC. (U.S.), Aviva Systems Biological Corporation (U.S.), Biocept, Inc. (U.S.), Fluxion Biosciences, Inc. (U.S.), Greiner Bio-One International GmbH (Austria) , Ikonisys (U.S.).
Market Developments
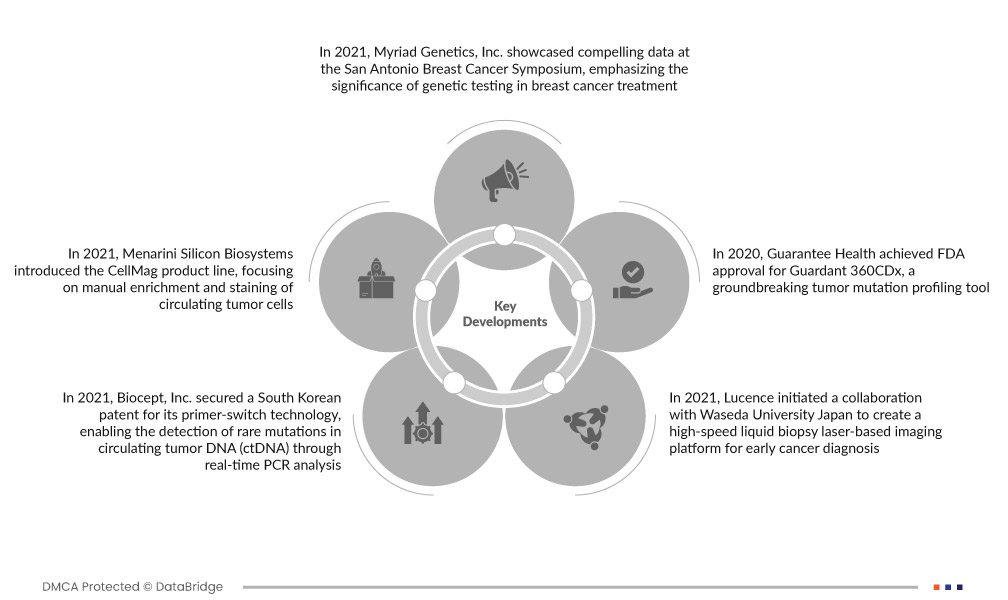
- In 2021, Myriad Genetics, Inc. showcased compelling data at the San Antonio Breast Cancer Symposium, emphasizing the significance of genetic testing in breast cancer treatment. This presentation not only enhances Myriad Genetics, Inc.'s credibility but also paves the way for the launch of innovative products. The integration of genetic testing advancements is anticipated to significantly contribute to the expansion of the liquid biopsy market, reinforcing its role in advancing cancer diagnosis and treatment
- In 2021, Menarini Silicon Biosystems introduced the CellMag product line, focusing on manual enrichment and staining of circulating tumor cells. This strategic expansion of their product offerings enhances Menarini Silicon Biosystems' market position and is poised to contribute significantly to the growth of the Circulating Tumor Cells (CTC) Liquid Biopsy Market by meeting evolving demands in the field of cancer diagnostics
- In 2021, Biocept, Inc. secured a South Korean patent for its primer-switch technology, enabling the detection of rare mutations in circulating tumor DNA (ctDNA) through real-time PCR analysis. This innovation allows the identification of rare cancer biomarkers in various bodily fluids, positioning Biocept, Inc. for revenue growth as it expands its capabilities in precision cancer diagnostics
- In 2021, Lucence initiated a collaboration with Waseda University Japan to create a high-speed liquid biopsy laser-based imaging platform for early cancer diagnosis. This innovative technology involves capturing images of circulating tumor cells (CTC) from blood samples, aiming to revolutionize early cancer detection by providing a rapid and efficient imaging solution for improved diagnostic precision
- In 2020, Guarantee Health achieved FDA approval for Guardant 360CDx, a groundbreaking tumor mutation profiling tool. This technology provides comprehensive genomic profiling by analyzing a simple blood sample, delivering results within seven days. The FDA approval underscores its efficacy, positioning Guardant 360CDx as a valuable asset in advancing precision medicine and cancer diagnostics.
Regional Analysis
Geographically, the countries covered in the global circulating tumor cells (CTC) liquid biopsy market report are U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
As per Data Bridge Market Research analysis:
North America is the dominant region in the global circulating tumor cells (CTC) liquid biopsy market during the forecast period 2023-2030
North America dominates the global circulating tumor cells (CTC) liquid biopsy market, primarily driven by the widespread adoption of liquid biopsy for cancer diagnosis. The region's increasing cancer cases contribute to its dominance, notably in the U.S. The preference for liquid biopsy as a diagnostic tool for cancer detection fosters the market's growth, positioning North America as a key player in the global liquid biopsy landscape.
Asia-Pacific is expected to dominate the global circulating tumor cells (CTC) liquid biopsy market in the forecast period 2023-2030
The Asia Pacific is expected to dominate the market, driven by rising disposable income, growing awareness of liquid biopsy techniques, and increased adoption of advanced diagnostic methods in hospitals, clinics, and diagnostic laboratories. Additionally, the expanding healthcare expenditure and the increased presence of hospitals and diagnostic centers contribute to the market's upward trajectory in Asia.
For more detailed information about the global circulating tumor cells (CTC) liquid biopsy market report, click here – https://www.databridgemarketresearch.com/reports/global-circulating-tumor-cells-ctc-liquid-biopsy-market