癌症是全球最主要的健康問題之一。它是高度已開發國家的首要死亡原因,也是發展中國家的第二大死因。醫療技術的工程應用的多樣化改進也對國家的經濟成長產生了相當大的影響。儘管封閉系統轉移裝置最初僅用於保護醫護人員在準備和管理危險藥物時免受接觸,但後來它們有助於減少腫瘤科環境中的表面污染。
訪問完整報告@ https://www.databridgemarketresearch.com/reports/global-closed-system-transfer-devices-market
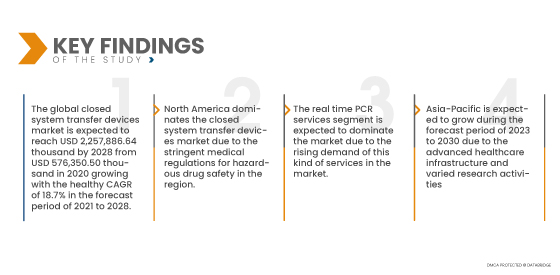
Data Bridge Market Research 分析稱,全球封閉系統轉移設備市場預計將從 2020 年的 576,350.50 千美元增至 2028 年的 2,257,886.64 千美元,在 2021 年至 2028 年的預測期內,複合年增長率將達到 18.7%。製藥業對封閉系統轉移設備的需求不斷增長,將為預測期內的市場成長提供潛在機會。
預計技術進步將推動市場成長率
醫療技術工程應用的重大改進對經濟成長產生了巨大影響。封閉系統轉移裝置 (CSTD) 可防止藥物洩漏到環境中,保護多名醫療保健專業人員免於接觸正在處理的藥物。最近,多項臨床研究進行了評估,並表明 CSTD 為醫護人員在藥物製備和管理過程中提高了安全性。
例如,
- BD 推出了 BD PhaSeal 系統,該系統有助於防止微生物入侵,並被眾多期刊記錄為藥瓶優化計劃的一部分,有助於減少藥物浪費
報告範圍和市場細分
報告指標
|
細節
|
預測期
|
2021年至2028年
|
基準年
|
2020
|
歷史歲月
|
2019(可訂製為2013年至2018年)
|
定量單位
|
收入(百萬美元)、銷售(單位)、定價(美元)
|
涵蓋的領域
|
類型(膜對膜系統和無針封閉系統轉移裝置)、組件(裝置和配件)、閉合機制(推轉系統、顏色對顏色對準系統、魯爾鎖系統和點擊鎖系統)、技術(基於隔膜的裝置、隔間裝置和空氣淨化/過濾裝置)、最終用戶(醫院、腫瘤中心和診所、門診手術中心、學術和研究機構)、零售渠道和研究機構
|
覆蓋國家
|
北美洲的美國、加拿大和墨西哥、德國、法國、英國、荷蘭、瑞士、比利時、俄羅斯、義大利、西班牙、土耳其、歐洲其他地區、中國、日本、印度、韓國、新加坡、馬來西亞、澳洲、泰國、印尼、菲律賓、亞太地區 (APAC) 的其他地區、沙烏地阿拉伯、阿聯酋、南非、埃及、以色列、中東和非洲 (MEA) 的其他地區、其他地區的歐洲地區
|
涵蓋的市場參與者
|
B. Braun Medical Inc.(美國)、ICU Medical, Inc(美國)、BD(美國)、Corvida Medical(美國)、YUKON MEDICAL(印度)、Caragen Ltd.(愛爾蘭)、Baxter(美國)、JMS North America Corporation(美國)、Vygon(法國)、Epic Medical Corporation(義大利)
|
報告涵蓋的數據點
|
除了對市場價值、成長率、細分、地理覆蓋範圍和主要參與者等市場情景的洞察之外,Data Bridge Market Research 策劃的市場報告還包括深度專家分析、患者流行病學、管道分析、定價分析和監管框架。
|
細分分析:
封閉系統傳輸設備市場分為六個顯著的部分,按類型、組件、封閉機制、技術、最終用戶和分銷管道劃分。
- 根據類型,封閉系統轉移裝置市場分為膜到膜系統和無針封閉系統轉移裝置。
預計膜對膜部分將主導封閉系統轉移設備市場
預計膜對膜部分將在 2021 年至 2028 年的預測期內以 18.2% 的複合年增長率在全球封閉系統轉移裝置中佔據主導地位,因為與無針封閉系統轉移裝置不同,它不易受到污染並且可以與現有設備一起使用。
- 根據組件,封閉系統傳輸設備市場已細分為設備和配件。裝置進一步分為小瓶接入裝置、注射器安全裝置、袋/管接入裝置、通風袋刺等。
預計組件類型的設備部分將主導封閉系統傳輸設備市場
由於其使用率高且與配件相比價格更高,預計該設備領域將在 2021 年至 2028 年預測期內以 19.0% 的複合年增長率在全球封閉系統傳輸設備中佔據主導地位
- 根據閉合機制,封閉系統傳輸設備市場細分為推轉系統、顏色對顏色對準系統、魯爾鎖系統和點擊鎖系統。由於其直覺的系統設計,無需組裝組件,預計點擊鎖定係統將在 2021 年至 2028 年的預測期內以 19.5% 的複合年增長率在全球封閉系統傳輸設備中佔據主導地位。
- 根據技術,封閉系統傳輸設備市場已細分為基於隔膜的設備、分隔式設備和空氣清潔/過濾設備。預計在 2021 年至 2028 年的預測期內,基於隔膜的設備領域將以 19.3% 的複合年增長率在全球封閉系統傳輸設備中佔據主導地位,因為它提高了操作的簡易性和安全性
- 根據最終用戶,封閉系統轉移設備市場分為醫院、腫瘤中心和診所、門診手術中心、學術和研究機構等。由於其操作簡便且安全,醫院部門預計將在 2021 年至 2028 年的預測期內以 19.4% 的複合年增長率在全球封閉系統轉移設備中佔據主導地位。
- 根據分銷管道,封閉系統傳輸設備市場分為直接招標和零售。預計零售領域將在 2021 年至 2028 年預測期內以 18.9% 的複合年增長率在全球封閉系統轉移設備中佔據主導地位,因為造成危險的可能性更大在醫院,借助封閉系統轉移設備,醫療保健專業人員可以防止自己接觸危險藥物。
主要參與者
Data Bridge Market Research 認為以下公司是封閉系統轉移設備市場的主要封閉系統轉移設備市場參與者,它們是 B. Braun Medical Inc.(美國)、ICU Medical, Inc(美國)、BD(美國)、Corvida Medical(美國)、YUKON MEDICAL(印度)、Caragen Ltd.(愛爾蘭)、Baxter(美國)、YUKON MEDICAL(印度)、Caragen Ltd.(愛爾蘭)、Baxter(美國)、JMS North America Corporation(法國)。
市場開發
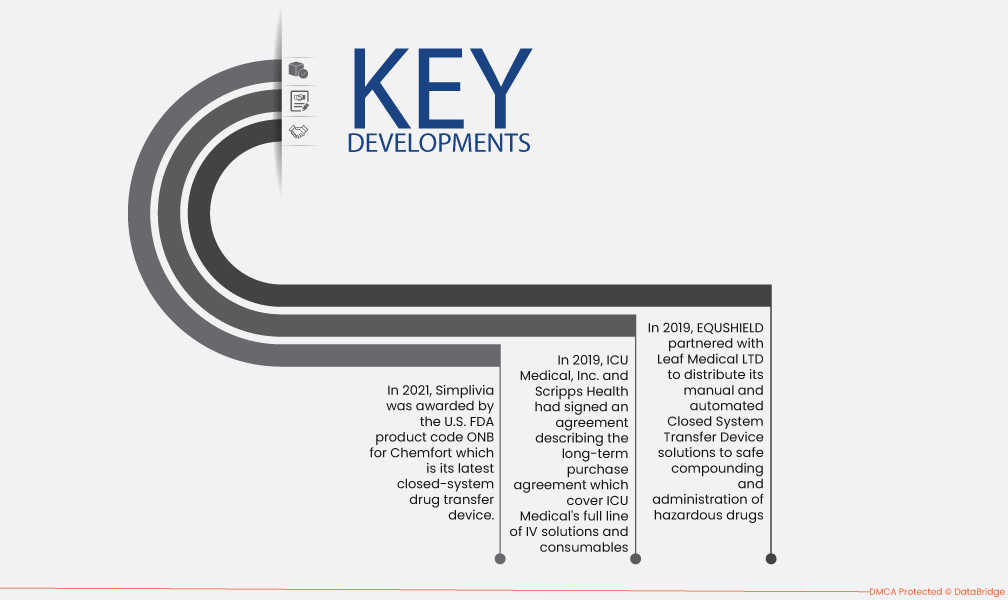
- 2021年,Simplivia最新的閉式系統藥物轉移裝置Chemfort榮獲美國FDA產品代碼ONB。它將有助於防止液體、液滴和氣溶膠逸出,並成功減少醫護人員接觸化療藥物的機會。
- 2019 年,ICU Medical, Inc. 與 Scripps Health 簽署了一項協議,描述了長期採購協議,該協議涵蓋 ICU Medical 的全系列 IV 溶液和耗材,以及帶有 ICU Medical MedNet IV 藥物安全軟體的 Plum 360 輸液系統。
- 2019 年,EQUSHIELD 與 Leaf Medical LTD 合作,分銷其手動和自動封閉系統轉移裝置解決方案,用於安全配製和管理危險藥物
區域分析
從地理上看,封閉系統轉移設備市場報告涵蓋的國家有:北美洲的美國、加拿大和墨西哥;歐洲的德國、法國、英國、荷蘭、瑞士、比利時、俄羅斯、義大利、西班牙、土耳其;歐洲的其他歐洲國家;中國、日本、印度、韓國、新加坡、馬來西亞、澳洲、泰國、印尼、菲律賓;亞太地區的其他亞太國家;
根據 Data Bridge 市場研究分析:
預測期內,北美是封閉系統轉移設備市場的主導地區
由於這種類型的使用增加,北美在封閉系統轉移設備市場佔據主導地位,因為它提供了一種更快、更有效的疫苗接種方式。此外,由於北美地區對危險藥物安全的醫療法規非常嚴格,美國在該地區佔據主導地位。
預計 2023 年至 2030 年預測期內,亞太地區將成為封閉系統轉移設備市場成長最快的地區
由於亞太地區擁有先進的醫療保健基礎設施和與腫瘤治療相關的研究活動,預計該地區將在 2023 年至 2030 年間實現成長。中國憑藉其醫學研究和技術進步在亞太地區佔據主導地位。
有關封閉系統傳輸設備市場 報告的更多詳細信息,請點擊此處 - https://www.databridgemarketresearch.com/reports/global-closed-system-transfer-devices-market