The rising incidence of surgical procedures and subsequent Intensive Care Unit (ICU) admissions has synergistically fuelled the demand for advanced hemodynamic monitoring. Surgical interventions, ranging from routine procedures to complex surgeries, often necessitate vigilant monitoring of a patient's hemodynamic status to ensure optimal outcomes. Hemodynamic monitoring involves continuously assessing cardiovascular parameters such as blood pressure, cardiac output, and fluid status, providing crucial insights into the patient's circulatory system during and after surgery.
The rise in chronic diseases, including diabetes, hypertension, and obesity, has led to an increased need for surgical procedures to manage and treat these conditions. For instance, individuals with diabetes may require surgeries related to complications such as peripheral artery disease or diabetic neuropathy.
In addition, as surgical interventions become more sophisticated, the need for accurate hemodynamic data becomes paramount, especially in procedures such as cardiac surgeries, organ transplants, and major orthopaedic surgeries. In critical care settings, especially ICUs, hemodynamic monitoring becomes crucial for managing patients with complex medical conditions or postoperative recovery. For instance, according to the NCBI article, the basics of hemodynamic monitoring have changed very little in recent years. The main goal of hemodynamic monitoring in critically ill patients remains the correct assessment of the cardiovascular system and its response to tissue oxygen demand.
Access Full Report @ https://www.databridgemarketresearch.com/reports/global-hemodynamic-monitoring-market
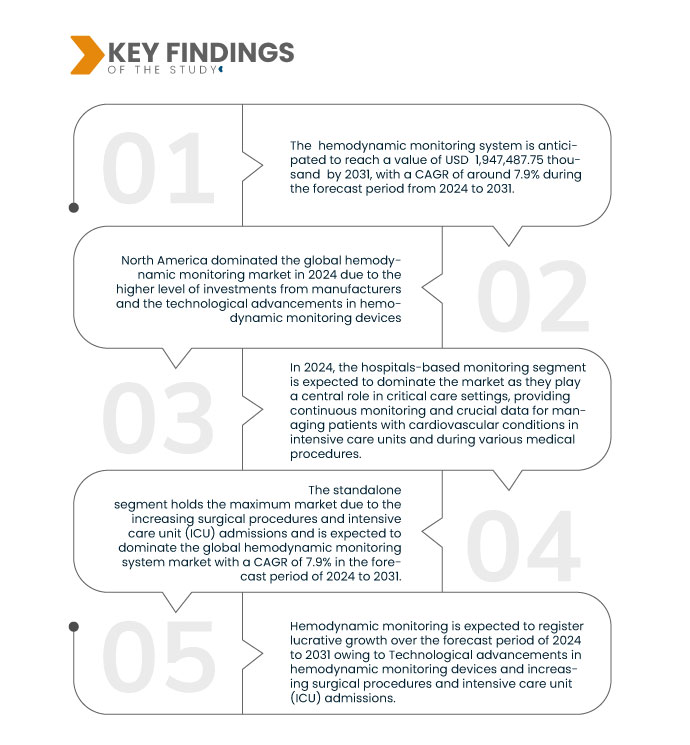
Data Bridge Market Research analyses that the Global Hemodynamic Monitoring Market is expected to grow with a CAGR of 7.2% in the forecast period from 2024 to 2031, and is expected to reach USD 3,839,125.07 thousand by 2031. In 2024, the Hemodynamic Monitoring Systems segment is expected to dominate the market due is rising incidences of cardiovascular disorders.
Key Findings of the Study
Integration of Hemodynamic Monitoring with Telemedicine
Integration with telemedicine represents a transformative advancement in healthcare, especially in the context of hemodynamic monitoring. The integration of hemodynamic monitoring with telemedicine represents a transformative approach to healthcare delivery, leveraging technology to provide real-time assessment and intervention for patients' cardiovascular health.
Integration with telemedicine is reshaping healthcare,
For instance,
Real-time Data Capture:
Hemodynamic monitoring involves continuously measuring cardiovascular parameters such as blood pressure, heart rate, and oxygen saturation. Wearable devices equipped with sensors capture these data points in real-time, providing a comprehensive picture of the patient's cardiovascular status.
Report Scope and Market Segmentation
|
Report Metric
|
Details
|
|
Forecast Period
|
2024 to 2031
|
|
Base Year
|
2023
|
|
Historic Years
|
2022 (Customizable to 2016-2021)
|
|
Quantitative Units
|
Revenue in USD Thousand
|
|
Segments Covered
|
Product (Hemodynamic Monitoring Systems, Vital Sign Monitors, Pulse Oximeters, Catheters and Consumables and Accessories), Type (Non-Invasive Hemodynamic Monitoring, Minimally Invasive Hemodynamic Monitoring and Invasive Hemodynamic Monitoring), Modality (Standalone, Table Top, Portable, Wearable and Other), Application (Hospitals-Based Monitoring, Laboratory-Based Monitoring and Home-Based Monitoring), Configuration (Automated and Manual), Age Group (Adult, Geriatric, and Pediatric), End User (Hospitals, Ambulatory Surgical Centers, Catheterization Labs, Nursing Homes, Homecare, Medical Institutions, Rehabilitation Centers, and Others), Distribution Channel (Offline and Online)
|
|
Countries Covered
|
U.S., Canada, Mexico, Germany, U.K., France, Russia, Italy, Spain, Turkey, Poland, Belgium, Netherlands, Switzerland, Denmark, Sweden, Norway, Finland, Rest of Europe, China, Japan, India, Australia, South Korea, New Zealand, Singapore, Thailand, Philippines, Malaysia, Indonesia, Vietnam, Taiwan, Rest of Asia-Pacific, Brazil, Argentina, Rest of South America, Saudi Arabia, UAE, South Africa, Egypt, Qatar, Kuwait, Oman, Bahrain and Rest of Middle East and Africa
|
|
Market Players Covered
|
Icumedical (U.S.), Teleflex Incorporated (U.S.), Koninklijke Philips N.V. (Netherlands), GE HealthCare (U.S.), Edwards Lifesciences Corporation (U.S.), Masimo (U.S.), Baxter (U.S.), Getinge AB (Sweden), CONTEC MEDICAL SYSTEMS CO.,LTD (China), Nihon Kohden Corporation (Japan), Merit Medical Systems (U.S.), Drägerwerk AG & Co. KGaA (Germany), OSYPKA MEDICAL (Germany), Deltex Medical (U.S.), SC, Schwarzer Cardiotek (Germany), Caretaker LLC. (U.S.), Uscom (Australia), NI-Medical (Israel), CNSystems Medizintechnik GmbH (Austria), and Argon Medical Devices (U.S.), among others.
|
|
Data Points Covered in the Report
|
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
|
Segment Analysis
The global hemodynamic monitoring market is categorized into eight notable segments based on product, type, modality, application, configuration, age group, end-user, and distribution channel.
- On the basis of product, the market is segmented into hemodynamic monitoring systems, vital sign monitors, pulse oximeters, catheters, and consumables and accessories
In 2024, the hemodynamic monitoring segment is expected to dominate the market
In 2024, the hemodynamic monitoring systems segment is expected to dominate the market with a 48.50% market share due to rising incidence of cardiovascular disorders.
- On the basis of type, the market is segmented into non-invasive hemodynamic monitoring, minimally invasive hemodynamic monitoring, and invasive hemodynamic monitoring
In 2024, the non-invasive hemodynamic monitoring segment is expected to dominate the market
In 2024, the non-invasive hemodynamic monitoring segment is expected to dominate the market with a 61.13% market share due to technological advancements in hemodynamic monitoring devices.
- On the basis of modality, the market is segmented into standalone, table top, portable, wearable, and other. In 2024, the standalone segment is expected to dominate the market with a 47.92% market share
- On the basis of application, the market is segmented into hospitals-based monitoring, laboratory-based monitoring, and home-based monitoring. In 2024, the hospital-based monitoring segment is expected to dominate the market with a 74.82% market share
- On the basis of configuration, the market is segmented into automated and manual. In 2024, the automated segment is expected to dominate the market with a 73.63% market share
- On the basis of age group, the market is segmented into adult, geriatric, and pediatric. In 2024, the female segment is expected to dominate the market with a 66.51% market share
- On the basis of end-user, the market is segmented into hospitals, ambulatory surgical centers, catheterization labs, nursing homes, homecare, medical institutions, rehabilitation centers, and others. In 2024, the hospitals & clinics segment is expected to dominate the market with a 66.64% market share
- On the basis of distribution channel, the market is segmented into offline and online. In 2024, the direct tenders segment is expected to dominate the market with a 84.04% market share
Major Players
Data Bridge Market Research analyses Edwards Lifesciences Corporation (U.S.), Masimo (U.S.), Getinge AB (Sweden), Baxter (U.S.), Teleflex Incorporated (U.S.) as the major companies in the global hemodynamic monitoring market.
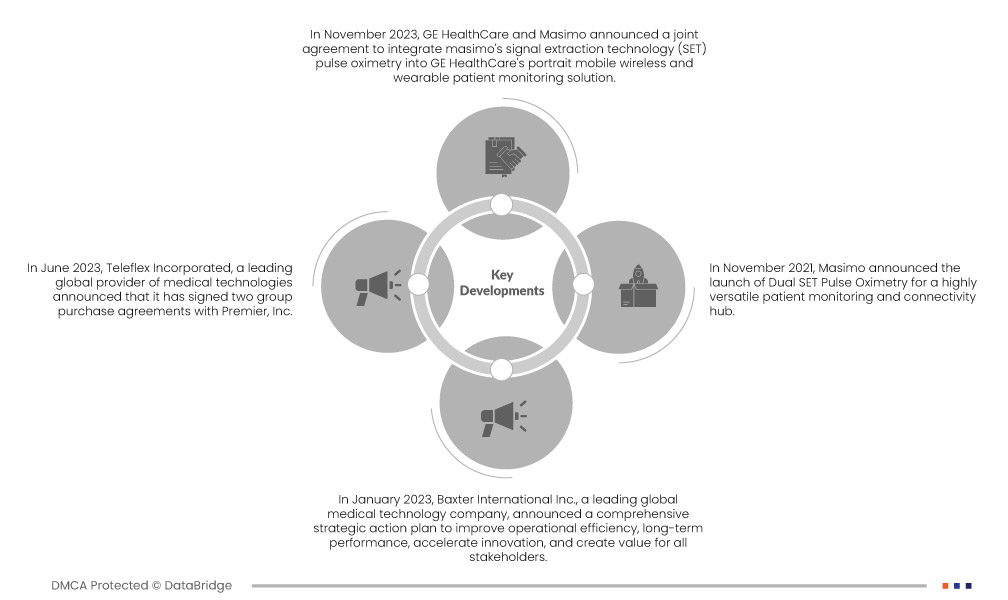
Market Developments
- In May 2024, Bristol Myers Squibb (NYSE: BMY) in partnership with Janssen Pharmaceuticals, Inc., one of the Janssen pharmaceutical companies of Johnson & Johnson (Janssen), announced that all three potential indications for milvexian, an investigational oral factor XIa (FXIa) inhibitorhas now received fast track designation from the U.S. Food and Drug Administration (FDA). The names include all three trials seeking indications in the Phase 3 Librexiadevelopment program (Librexia HEMODYNAMIC MONITORING, Librexia ACS, and Librexia AF), all dosed patients. The Librexia program is unparalleled as a comprehensive clinical development program for FXIa, providing extensive data from nearly 50,000 patients. This opens the door to a whole new range of currently neglected patients due to the risk of bleeding
- In January 2024, Penumbra, Inc., a global healthcare company focused on innovative therapies, announced the U.S. Food and Drug Administration (FDA) approval and launch of Lightning Flash, the most advanced and powerful mechanical thrombectomy system on the market. Lightning Flash features Penumbra's new Lightning Intelligent Aspiration technology, which now has two clot detection algorithms. Combined with innovative catheter technology, the Lightning Flash is designed to quickly remove large blood clots from the body, including venous embolism and pulmonary embolism (PE). This launch will help the company expand its product portfolio because the advanced results of this new technology are exceptionally traceable, and it has the unique ability to differentiate flowing blood from clots
- In July 2024, Roche announced a new partnership with Alnylam to develop and commercialize zilebesir, an investigational RNAi therapy currently in Phase 2 for treating high blood pressure. This collaboration combines Alnylam's proven experience in RNAi therapy with Roche's global commercial reach, commitment to innovation, and desire to change the landscape for patients with severe cardiovascular disease
- In February 2021, Sanofi’s Plavix (clopidogrel) got approval by the European Commission for its use in combination with aspirin in adult patients with moderate or high-risk transient ischemic attack (TIA) (ABCD2 score ≥4) or mild ischemic Hemodynamic Monitoring (IS). Twenty-four hours of a TIA or IS event. In this new indication, use can be continued for 21 days, followed by long-term single antiplatelet therapy. The additional indication is based on the results of a two-investigator-initiated, double-blind, randomized, placebo-controlled phase 3 study involving more than 10,000 patients. Studies have shown that the combination of clopidogrel and aspirin started within 24 hours is better than aspirin in reducing the risk of subsequent Hemodynamic Monitoring and has a generally acceptable safety profile. This new approval demonstrates companies unwavering commitment to advancing cardiovascular care
- In September 2020, Daiichi Sankyo Company Limited announced that it had submitted a supplemental application in Japan for the extended approval of the anticoagulant edoxaban (edoxaban benzoate hydrate) in elderly patients with nonvalvular regurgitation and severe bleeding risk. This application is based on the results of a Japanese Phase 3 clinical trial (ELDERCARE-AF trial) in 984 patients with non-valvular atrial fibrillation who are at least 80 years old and have a high risk of bleeding and are not suitable for other available anticoagulant therapies. Daiichi Sankyo plans to contribute to treating elderly patients with non-valvular atrial fibrillation by offering a new treatment option
Regional Analysis
Geographically, the countries covered in the global hemodynamic monitoring market report are the U.S., Canada, Mexico, Germany, U.K., France, Russia, Italy, Spain, Turkey, Poland, Belgium, Netherlands, Switzerland, Denmark, Sweden, Norway, Finland, Rest of Europe, China, Japan, India, Australia, South Korea, New Zealand, Singapore, Thailand, Philippines, Malaysia, Indonesia, Vietnam, Taiwan, Rest of Asia-Pacific, Brazil, Argentina, Rest of South America, Saudi Arabia, UAE, South Africa, Egypt, Qatar, Kuwait, Oman, Bahrain and Rest of Middle East and Africa as per Data Bridge Market Research analysis:
North America is expected to dominate the global hemodynamic monitoring market
North America is expected to dominate the market owing to the high demand for hemodynamic monitoring in the region and the rise in healthcare expenditure. North America will continue to dominate the hemodynamic monitoring market in terms of market share and market revenue and will continue to flourish its dominance during the forecast period.
Asia-Pacific is estimated to be the fastest-growing region in the global hemodynamic monitoring market
Asia-Pacific is estimated to be the fastest-growing region in the market for the forecast period, due to the growing adoption of advanced technology and the launch of new products in this region.
For more detailed information about the global hemodynamic monitoring market report, click here – https://www.databridgemarketresearch.com/reports/global-hemodynamic-monitoring-market