Согласно отчету "Mycoplasma genitalium Urethritis: Prevalence and Drug-Resistance Patterns", опубликованному в марте 2020 года, Mycoplasma gentitalium является одной из основных причин мужского уретрита, а согласно исследованию, проведенному на 914 участниках, 28,7% населения имели инфекцию Mycoplasma genitalium (MG). Согласно новостям за февраль 2019 года, Mycoplasma genitalium была выделена из прямой кишки и анального канала пациентов с болезнью Крона, что подразумевает, что Mycoplasma также ответственна за распространенные желудочно-кишечные инфекции.
Полный отчет доступен по адресу https://databridgemarketresearch.com/reports/global-mycoplasma-testing-in-clinical-market
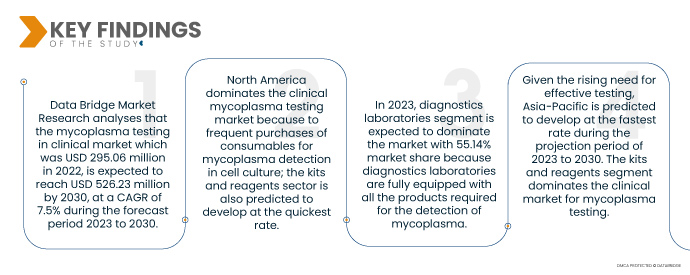
Data Bridge Market Research анализирует, что рынок тестирования микоплазмы на клиническом уровне , который в 2022 году составил 295,06 млн долларов США, как ожидается, достигнет 526,23 млн долларов США к 2030 году при среднегодовом темпе роста 7,5% в прогнозируемый период с 2023 по 2030 год. Были разработаны высокочувствительные ПЦР и кПЦР, повышающие специфичность тестирования более чем 90 видов микоплазм. Тест включает в себя различные товары, такие как ридеры, анализы, инструменты, наборы и реагенты, все из которых способствуют росту рынка. Огромное количество участников рынка вовлечено в производство устройств и расходных материалов, используемых для идентификации различных видов микоплазм, что прокладывает путь к расширению рынка.
Ожидается , что рост числа хронических заболеваний будет способствовать темпам роста рынка.
В наши дни растет число различных инфекционных заболеваний из-за плохой гигиены и ряда других факторов. Известно, что микоплазма вызывает множество заболеваний, поражая различные части тела. Микоплазма связана с уретритом, болезнью Крона, пневмонией и неврологическими заболеваниями, среди прочего. Поскольку микоплазма является основной причиной многих заболеваний, необходимость в клинических испытаниях растет для предоставления надлежащего лечения в нужное время. В результате, растущее число заболеваний стимулирует расширение мирового рынка испытаний на микоплазму.
Область отчета и сегментация рынка
Отчет Метрика
|
Подробности
|
Прогнозируемый период
|
2023-2030
|
Базовый год
|
2022
|
Исторические годы
|
2021 (можно настроить на 2015-2020)
|
Количественные единицы
|
Доход в млн. долл. США, объемы в единицах, цены в долл. США
|
Охваченные сегменты
|
Продукция (наборы и реагенты, инструменты, услуги), Метод (методы микробного культивирования/прямой анализ, полимеразная цепная реакция, ИФА, окрашивание ДНК/косвенный анализ, ферментативные методы), Область заболевания (респираторная, урогенитальная, желудочно-кишечная, опорно-двигательная, сердечно-сосудистая и другие), Конечный пользователь (диагностические лаборатории, больницы)
|
Страны, охваченные
|
США, Канада и Мексика в Северной Америке, Германия, Франция, Великобритания, Нидерланды, Швейцария, Бельгия, Россия, Италия, Испания, Турция, Остальная Европа в Европе, Китай, Япония, Индия, Южная Корея, Сингапур, Малайзия, Австралия, Таиланд, Индонезия, Филиппины, Остальная часть Азиатско-Тихоокеанского региона (APAC) в Азиатско-Тихоокеанском регионе (APAC), Саудовская Аравия, ОАЭ, Южная Африка, Египет, Израиль, Остальной Ближний Восток и Африка (MEA) как часть Ближнего Востока и Африки (MEA), Бразилия, Аргентина и Остальная часть Южной Америки как часть Южной Америки
|
Охваченные участники рынка
|
AB ANALITICA srl (Италия), bioMérieux SA (Франция), ELITechGroup (Франция), Liofilchem Srl (Италия), Agilent Technologies, Inc. (США), PromoCell GmbH (Германия), F. Hoffmann-La Roche Ltd (Швейцария), OSANG Healthcare (Южная Корея), Sacace Biotechnologies Srl (Италия), Lonza (Швейцария), Merck KGaA (Германия), Seegene Inc. (Южная Корея), Clongen Laboratories, LLC (США), Bio-Rad Laboratories, Inc. (США), Charles River Laboratories (США), Bionique Testing Laboratories, Inc. (США) и ZEAKON Diagnostics (США)
|
Данные, отраженные в отчете
|
Помимо таких рыночных данных, как рыночная стоимость, темпы роста, сегменты рынка, географический охват, участники рынка и рыночный сценарий, рыночный отчет, подготовленный командой Data Bridge Market Research, включает в себя углубленный экспертный анализ, эпидемиологию пациентов, анализ воронки продаж, анализ цен и нормативную базу.
|
Анализ сегмента:
Глобальное клиническое тестирование на микоплазму подразделяется на четыре основных сегмента, которые различаются по видам продукции, методикам, областям заболевания и конечным пользователям.
- На основе продуктов тестирование микоплазмы на клиническом рынке сегментируется на наборы и реагенты, инструменты и услуги. Ожидается, что в 2023 году сегмент наборов и реагентов будет доминировать на рынке с долей рынка 54,31%, поскольку наборы и реагенты неоднократно приобретаются как продукты для обнаружения микоплазмы.
- На основе техники тестирование микоплазмы на клиническом рынке сегментируется на методы микробной культуры/прямой анализ, полимеразную цепную реакцию, ИФА, окрашивание ДНК/косвенный анализ, ферментативные методы. Ожидается, что в 2023 году сегмент методов микробной культуры/прямого анализа будет доминировать на рынке с долей рынка 36,42%, поскольку эти методы являются экономически эффективными и высокоспецифичными при получении положительного результата.
- На основе области заболевания тестирование на микоплазму на клиническом рынке сегментируется на респираторные, урогенитальные, желудочно-кишечные, сердечно-сосудистые, опорно-двигательного аппарата и др. Ожидается, что в 2023 году респираторный сегмент будет доминировать на рынке с 36,37%, поскольку микоплазма поражает детей и взрослых с легкой и тяжелой пневмонией.
Респираторный сегмент будет доминировать в сегменте заболеваний при тестировании микоплазмы на клиническом рынке.
Респираторный сегмент станет доминирующим сегментом в области заболеваний с долей рынка около 37,00%. Это связано с растущим числом мероприятий по развитию инфраструктуры на рынке, особенно в развивающихся экономиках. Кроме того, рост и расширение отрасли здравоохранения по всему миру еще больше поддержит рост этого сегмента.
- На основе конечного пользователя тестирование микоплазмы на клиническом рынке сегментируется на больницы и диагностические лаборатории. Ожидается, что в 2023 году сегмент диагностических лабораторий будет доминировать на рынке с долей рынка 55,14%, поскольку диагностические лаборатории полностью оснащены всеми продуктами, необходимыми для обнаружения микоплазмы, и имеют квалифицированных специалистов.
Сегмент диагностических лабораторий будет доминировать в сегменте конечного пользователя тестирования микоплазмы на клиническом рынке.
Сегмент диагностических лабораторных центров станет доминирующим сегментом конечного пользователя. Это связано с растущим числом диагностических лабораторий на рынке, особенно в развивающихся экономиках. Кроме того, рост и расширение услуг по развитию исследований в глобальном масштабе еще больше поддержит рост этого сегмента.
Основные игроки
Data Bridge Market Research выделяет следующие компании в качестве основных игроков рынка: AB ANALITICA srl (Италия), bioMérieux SA (Франция), ELITechGroup (Франция), Liofilchem Srl (Италия), Agilent Technologies, Inc. (США), PromoCell GmbH (Германия), F. Hoffmann-La Roche Ltd (Швейцария), OSANG Healthcare (Южная Корея), Sacace Biotechnologies Srl (Италия), Lonza (Швейцария), Merck KGaA (Германия), Seegene Inc. (Южная Корея), Clongen Laboratories, LLC (США), Bio-Rad Laboratories, Inc. (США), Charles River Laboratories (США), Bionique Testing Laboratories, Inc. (США) и ZEAKON Diagnostics (США).
Развитие рынка
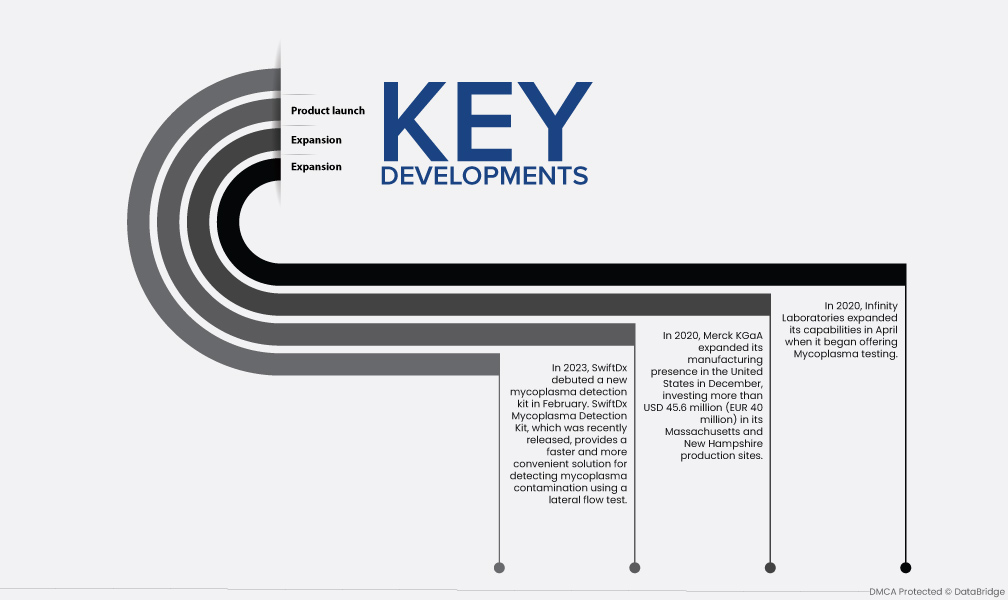
- В феврале 2023 года SwiftDx дебютировал с новым набором для обнаружения микоплазмы. Недавно выпущенный набор для обнаружения микоплазмы SwiftDx обеспечивает более быстрое и удобное решение для обнаружения заражения микоплазмой с помощью теста с латеральным потоком.
- В декабре 2020 года компания Merck KGaA расширила свое производственное присутствие в Соединенных Штатах, инвестировав более 45,6 млн долларов США (40 млн евро) в свои производственные площадки в Массачусетсе и Нью-Гемпшире. Площадки были спроектированы для производства различных биофармацевтических производственных товаров, а также для увеличения производственных мощностей компании.
- В 2020 году Infinity Laboratories расширила свои возможности в апреле, начав предлагать тестирование на микоплазму. Возможности ведущих игроков рынка улучшатся в результате внедрения ключевых стратегий, таких как расширение, партнерство, поглощение и другие, что приведет к расширению рынка.
Региональный анализ
Географически в отчете о рынке рассматриваются следующие страны: США, Канада и Мексика в Северной Америке, Германия, Франция, Великобритания, Нидерланды, Швейцария, Бельгия, Россия, Италия, Испания, Турция, остальные страны Европы в Европе, Китай, Япония, Индия, Южная Корея, Сингапур, Малайзия, Австралия, Таиланд, Индонезия, Филиппины, остальные страны Азиатско-Тихоокеанского региона (APAC) в Азиатско-Тихоокеанском регионе (APAC), Саудовская Аравия, ОАЭ, Южная Африка, Египет, Израиль, остальные страны Ближнего Востока и Африки (MEA) как часть Ближнего Востока и Африки (MEA), Бразилия, Аргентина и остальные страны Южной Америки как часть Южной Америки.
Согласно анализу Data Bridge Market Research:
Северная Америка будет доминирующим регионом на рынке тестирования микоплазмы на клиническом рынке в прогнозируемый период с 2023 по 2030 год.
Северная Америка доминирует на рынке клинического тестирования на микоплазму из-за частых закупок расходных материалов для обнаружения микоплазмы в клеточных культурах; также прогнозируется, что сектор наборов и реагентов будет развиваться наиболее быстрыми темпами.
По оценкам, Азиатско-Тихоокеанский регион станет регионом с самыми быстрыми темпами роста рынка клинических исследований на микоплазму в прогнозируемый период с 2023 по 2030 год.
Учитывая растущую потребность в эффективном тестировании, прогнозируется, что Азиатско-Тихоокеанский регион будет развиваться наиболее быстрыми темпами в прогнозируемый период с 2023 по 2030 год. Сегмент наборов и реагентов доминирует на клиническом рынке тестирования на микоплазму.
Для получения более подробной информации об отчете о клиническом рынке тестирования на микоплазму нажмите здесь – https://www.databridgemarketresearch.com/reports/global-mycoplasma-testing-in-clinical-market