وفقًا لتقرير المؤسسة الدولية لهشاشة العظام، تراوحت نسبة الإصابة بهشاشة العظام لدى الرجال فوق سن الخمسين من 5.7% (سلوفاكيا) إلى 6.9% (السويد) في أوروبا. وتراوحت نسبة الإصابة بهشاشة العظام لدى النساء من 19.3% (قبرص) إلى 23.4% (إيطاليا). هشاشة العظام مرض مزمن تُصبح فيه العظام هشة وقابلة للكسر بسبب نقص فيتامين د. إذا كان الشخص مصابًا بهشاشة العظام، فسيكون أكثر عرضة لكسور العظام غير المتوقعة. وذكرت بيانات منظمة الصحة العالمية (WHO) أنه من المتوقع أن يُصاب حوالي 200 مليون شخص بهشاشة العظام في عام 2020. وعلى الرغم من أن هشاشة العظام تصيب الإناث والذكور على حد سواء، إلا أن النساء أكثر عرضة للإصابة بهشاشة العظام من الرجال.
الوصول إلى التقرير الكامل على https://www.databridgemarketresearch.com/reports/global-orthopedic-surgical-energy-devices-market
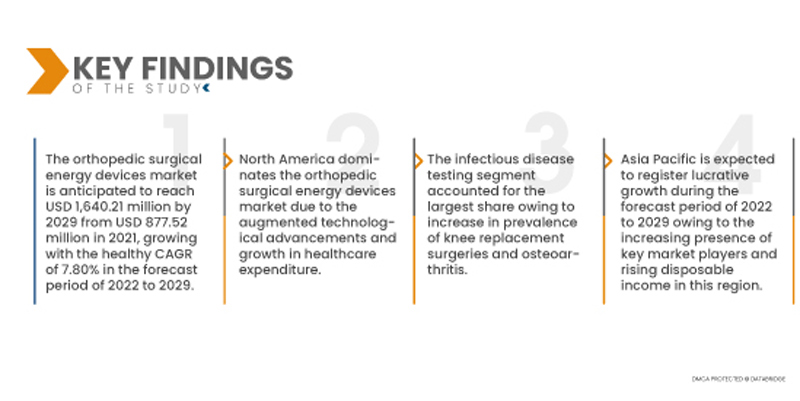
تحلل شركة Data Bridge Market Research أن سوق أجهزة الطاقة الجراحية العظمية من المتوقع أن يصل إلى 1،640.21 مليون دولار أمريكي بحلول عام 2029 من 877.52 مليون دولار أمريكي في عام 2021، وينمو بمعدل نمو سنوي مركب صحي بنسبة 7.80٪ في الفترة المتوقعة من 2022 إلى 2029. سيوفر الطلب المتزايد على أجهزة الطاقة الجراحية العظمية عبر القطاع الطبي فرصًا محتملة لنمو السوق في الفترة المتوقعة.
من المتوقع أن يؤدي النمو السكاني لكبار السن إلى دفع معدل نمو السوق
ساهم النمو الهائل في عدد المرضى، نتيجةً لتزايد أعداد كبار السن عالميًا، بشكل كبير في نمو سوق أجهزة الطاقة الجراحية العظمية خلال فترة التوقعات. ووفقًا لتقرير مكتب المراجع السكانية، ارتفعت نسبة سكان العالم الذين تزيد أعمارهم عن 65 عامًا إلى 9% في عام 2019. وبلغ عدد سكان العالم الذين تبلغ أعمارهم 65 عامًا فأكثر حوالي 703 ملايين نسمة في عام 2019. وقد أدى هذا النمو في أعداد كبار السن إلى زيادة الطلب على الرعاية الطبية وزيادة الإنفاق على الرعاية الصحية. وقد أدت هذه العوامل إلى زيادة الطلب على منتجات أجهزة الطاقة الجراحية العظمية، ومن المتوقع أن تدفع نمو السوق خلال فترة التوقعات.
نطاق التقرير وتقسيم السوق
مقياس التقرير
|
تفاصيل
|
فترة التنبؤ
|
من 2022 إلى 2029
|
سنة الأساس
|
2021
|
السنوات التاريخية
|
2020 (قابلة للتخصيص من 2014 إلى 2019)
|
الوحدات الكمية
|
الإيرادات بالملايين من الدولارات الأمريكية، والحجم بالوحدات، والتسعير بالدولار الأمريكي
|
القطاعات المغطاة
|
المنتج (القطع اليدوية والملحقات)، التكنولوجيا (الإشعاع، الترددات الراديوية، الموجات فوق الصوتية، الميكروويف، وغيرها)، التطبيق (الورك، الركبة)، المستخدم النهائي (المستشفيات والعيادات، مراكز الجراحة الخارجية، وغيرها)،
|
الدول المغطاة
|
الولايات المتحدة وكندا والمكسيك في أمريكا الشمالية، ألمانيا، فرنسا، المملكة المتحدة، هولندا، سويسرا، بلجيكا، روسيا، إيطاليا، إسبانيا، تركيا، بقية دول أوروبا في أوروبا، الصين، اليابان، الهند، كوريا الجنوبية، سنغافورة، ماليزيا، أستراليا، تايلاند، إندونيسيا، الفلبين، بقية دول آسيا والمحيط الهادئ (APAC) في منطقة آسيا والمحيط الهادئ (APAC)، المملكة العربية السعودية، الإمارات العربية المتحدة، جنوب أفريقيا، مصر، إسرائيل، بقية دول الشرق الأوسط وأفريقيا (MEA) كجزء من الشرق الأوسط وأفريقيا (MEA)، البرازيل والأرجنتين وبقية دول أمريكا الجنوبية كجزء من أمريكا الجنوبية
|
الجهات الفاعلة في السوق المغطاة
|
زيمر بيوميت (الولايات المتحدة)، سميث آند نيفيو (المملكة المتحدة)، ميدترونيك (أيرلندا)، سترايكر (الولايات المتحدة)، براون ميلسونجن إيه جي (ألمانيا)، نوفاسيف (الولايات المتحدة)، دي جي أو (الولايات المتحدة)، معهد سترومان إيه جي (سويسرا)، أوستيم إمبلانت المحدودة (كوريا الجنوبية)، نارانج ميديكال ليمتد (الهند)، غلوبس ميديكال (الولايات المتحدة)، آرثركس (الولايات المتحدة)، كونميد كوربوريشن (الولايات المتحدة)، إنتيغرا لايف ساينسز كوربوريشن (الولايات المتحدة)، آر تي آي سرجيكال (الولايات المتحدة)، دبليو إل جور آند أسوشيتس (الولايات المتحدة)، كورين جروب (المملكة المتحدة)، جونسون آند جونسون سيرفيسز (الولايات المتحدة).
|
نقاط البيانات التي يغطيها التقرير
|
بالإضافة إلى الرؤى حول سيناريوهات السوق مثل القيمة السوقية ومعدل النمو والتجزئة والتغطية الجغرافية واللاعبين الرئيسيين، فإن تقارير السوق التي تم تنظيمها بواسطة Data Bridge Market Research تتضمن أيضًا تحليلًا متعمقًا من الخبراء وعلم الأوبئة للمرضى وتحليل خطوط الأنابيب وتحليل التسعير والإطار التنظيمي.
|
تحليل القطاعات:
يتم تقسيم سوق أجهزة الطاقة الجراحية العظمية على أساس المنتج والتكنولوجيا والتطبيق والمستخدم النهائي.
- على أساس المنتج، يتم تقسيم سوق أجهزة الطاقة الجراحية العظمية العالمية إلى قطع يدوية وملحقات.
من المتوقع أن يهيمن قطاع قطع اليد من قطاع المنتجات على سوق أجهزة الطاقة الجراحية العظمية
من المتوقع أن تهيمن شريحة القطع اليدوية على سوق أجهزة الطاقة الجراحية العظمية العالمية بمعدل نمو سنوي مركب قدره 8.3٪ في الفترة المتوقعة من 2022 إلى 2029 بسبب الدقة الأفضل وسهولة الاستخدام واستخدام تقنية الترددات الراديوية في القطع اليدوية، والتي تنقل الطاقة للتخثر وإغلاق العظام والأنسجة الرخوة.
- على أساس التكنولوجيا، يتم تقسيم سوق أجهزة الطاقة الجراحية العظمية العالمية إلى الترددات الراديوية والإشعاع والموجات فوق الصوتية والميكروويف وغيرها.
من المتوقع أن يهيمن قطاع الترددات الراديوية في قطاع التكنولوجيا على سوق أجهزة الطاقة الجراحية العظمية
من المتوقع أن تهيمن شريحة الترددات الراديوية على سوق أجهزة الطاقة الجراحية العظمية العالمية بمعدل نمو سنوي مركب قدره 9.1٪ في الفترة المتوقعة من 2022 إلى 2029 لأن الترددات الراديوية هي تقنية قليلة التوغل تهدف إلى علاج الألم المزمن في مرض هشاشة العظام وآمنة للاستخدام مع الحد الأدنى من الكدمات.
- بناءً على التطبيق، يُقسّم سوق أجهزة الطاقة الجراحية العظمية العالمية إلى جراحات الورك والركبة وغيرها. في عام 2022، من المتوقع أن يهيمن قطاع اختبار الأمراض المعدية على سوق أجهزة الطاقة الجراحية العظمية العالمية بمعدل نمو سنوي مركب قدره 8.2% خلال الفترة المتوقعة من 2022 إلى 2029، وذلك بفضل زيادة انتشار جراحات استبدال الركبة وهشاشة العظام. على سبيل المثال، أُجريت حوالي مليون جراحة استبدال ركبة في عام 2020 في جميع أنحاء الولايات المتحدة.
- بناءً على المستخدم النهائي، يُقسّم سوق أجهزة الطاقة الجراحية العظمية العالمية إلى مستشفيات وعيادات، ومراكز جراحية متنقلة، وغيرها. في عام 2022، من المتوقع أن يهيمن قطاع المستشفيات والعيادات على سوق أجهزة الطاقة الجراحية العظمية العالمية بمعدل نمو سنوي مركب قدره 8.1% خلال الفترة المتوقعة من 2022 إلى 2029، وذلك بفضل توافر أجهزة الطاقة الجراحية العظمية المتقدمة، وزيادة عدد المرضى الذين يُدخلون المستشفيات، وارتفاع الدخل المتاح للتصرف.
- بناءً على قنوات التوزيع، يُقسّم سوق أجهزة الطاقة الجراحية العظمية العالمية إلى قسمين: قسم المناقصات المباشرة وقسم الموزعين الخارجيين. في عام 2022، من المتوقع أن يهيمن قطاع المناقصات المباشرة على سوق أجهزة الطاقة الجراحية العظمية العالمية بمعدل نمو سنوي مركب قدره 8.0% خلال الفترة المتوقعة من 2022 إلى 2029، وذلك بفضل زيادة الاتفاقيات التعاقدية بين الموزعين والمصنعين لأجهزة الطاقة الجراحية العظمية، وتكافؤ الفرص في عملية المناقصات.
اللاعبون الرئيسيون
تعترف شركة Data Bridge Market Research بالشركات التالية باعتبارها اللاعبين الرئيسيين في سوق أجهزة الطاقة الجراحية العظمية Zimmer Biomet (الولايات المتحدة)، Smith & Nephew (المملكة المتحدة)، Medtronic (أيرلندا)، Stryker (الولايات المتحدة)، Braun Melsungen AG (ألمانيا)، NuVasive، Inc. (الولايات المتحدة)، DJO، LLC (الولايات المتحدة)، Institut Straumann AG (سويسرا)، OSSTEM IMPLANT CO.، LTD. (كوريا الجنوبية)، Narang Medical Limited (الهند)، Globus Medical (الولايات المتحدة)، Arthrex، Inc. (الولايات المتحدة).
تطوير السوق
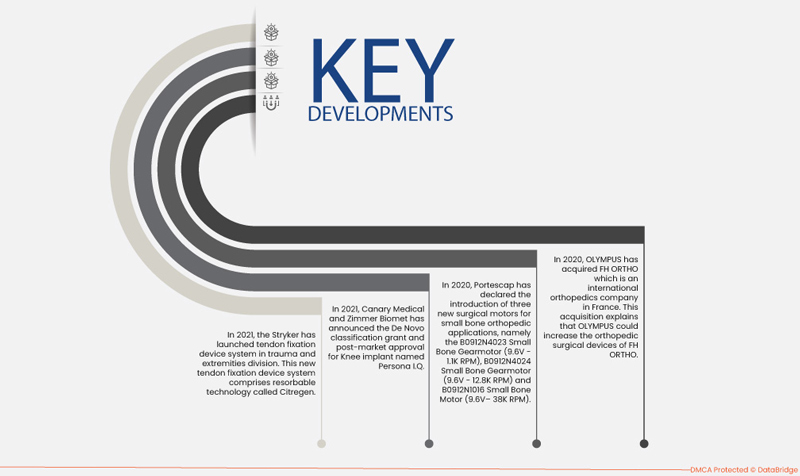
- في عام ٢٠٢١، أطلقت شركة سترايكر نظام تثبيت الأوتار في قسم الإصابات والأطراف. يعتمد هذا النظام الجديد على تقنية سيتريجين القابلة للامتصاص، وهي تقنية مُحسّنة لتطبيقات جراحة العظام. سيُمثل هذا الإطلاق نقلة نوعية في مجموعة منتجات قسم الإصابات والأطراف.
- في عام 2021، أعلنت شركة Canary Medical وZimmer Biomet عن منحة تصنيف De Novo والموافقة بعد التسويق لزرع الركبة المسمى Persona IQ. هذا الدعم من إدارة الغذاء والدواء من شأنه أن يؤدي إلى إضافة منتج جديد في توسيع خط أعمال العظام.
- في عام 2020، أعلنت شركة Portescap عن تقديم ثلاثة محركات جراحية جديدة لتطبيقات تقويم العظام الصغيرة، وهي B0912N4023 Small Bone Gearmotor (9.6V - 1.1K RPM)، وB0912N4024 Small Bone Gearmotor (9.6V - 12.8K RPM) وB0912N1016 Small Bone Motor (9.6V– 38K RPM).
- في عام ٢٠٢٠، استحوذت شركة أوليمبوس على شركة FH ORTHO، وهي شركة عالمية متخصصة في جراحة العظام في فرنسا. يُفسر هذا الاستحواذ قدرة أوليمبوس على زيادة عدد الأجهزة الجراحية العظمية التي تنتجها شركة FH ORTHO. ستتمكن هذه الشركة من تطوير جراحة العظام طفيفة التوغل في الدول النامية.
التحليل الإقليمي
جغرافيًا، البلدان التي يغطيها تقرير سوق أجهزة الطاقة الجراحية العظمية هي الولايات المتحدة وكندا والمكسيك في أمريكا الشمالية وألمانيا وفرنسا والمملكة المتحدة وهولندا وسويسرا وبلجيكا وروسيا وإيطاليا وإسبانيا وتركيا وبقية أوروبا في أوروبا والصين واليابان والهند وكوريا الجنوبية وسنغافورة وماليزيا وأستراليا وتايلاند وإندونيسيا والفلبين وبقية دول آسيا والمحيط الهادئ (APAC) في منطقة آسيا والمحيط الهادئ (APAC) والمملكة العربية السعودية والإمارات العربية المتحدة وجنوب إفريقيا ومصر وإسرائيل وبقية دول الشرق الأوسط وأفريقيا (MEA) كجزء من الشرق الأوسط وأفريقيا (MEA) والبرازيل والأرجنتين وبقية دول أمريكا الجنوبية كجزء من أمريكا الجنوبية
وفقًا لتحليل Data Bridge Market Research:
أمريكا الشمالية هي المنطقة المهيمنة في سوق أجهزة الطاقة الجراحية العظمية خلال الفترة المتوقعة من 2022 إلى 2029
من المتوقع أن تهيمن أمريكا الشمالية على السوق، وأن تشهد نموًا ملحوظًا بفضل التطورات التكنولوجية المتسارعة في هذه المنطقة. وستواصل أمريكا الشمالية هيمنتها على سوق أجهزة الطاقة الجراحية العظمية من حيث الإيرادات والحصة السوقية، وستواصل تعزيز هيمنتها في السنوات القادمة بفضل تنامي الإنفاق على الرعاية الصحية.
من المتوقع أن تكون منطقة آسيا والمحيط الهادئ أسرع المناطق نموًا في سوق أجهزة الطاقة الجراحية العظمية خلال الفترة المتوقعة من 2022 إلى 2029
من المرجح أن تسجل منطقة آسيا والمحيط الهادئ أعلى معدل نمو خلال الفترة المتوقعة من 2022 إلى 2029، نظرًا لتزايد انتشار كبار السن في الهند والصين واليابان. علاوة على ذلك، من المتوقع أن يعزز الحضور المتزايد للاعبين الرئيسيين في السوق وارتفاع الدخل المتاح معدل نمو السوق في هذه المنطقة.
لمزيد من المعلومات التفصيلية حول تقرير سوق أجهزة الطاقة الجراحية العظمية ، انقر هنا - https://www.databridgemarketresearch.com/reports/global-orthopedic-surgical-energy-devices-market