Ovarian cancer is a type of cancer that starts in the ovaries, which are two small organs located on either side of the uterus in the female pelvis. The ovaries produce eggs and hormones. Ovarian cancer is the fifth leading cause of cancer death among women, and the most common type of gynecologic cancer. It is estimated that about 22,240 women in the United States will be diagnosed with ovarian cancer in 2023, and about 14,070 women will die from the disease. The symptoms of ovarian cancer are often vague and non-specific, which can make it difficult to diagnose early.
Access Full Report @ https://www.databridgemarketresearch.com/reports/global-ovarian-cancer-diagnostics-market
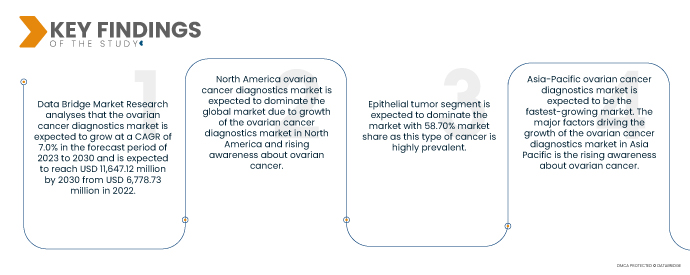
Data Bridge Market Research analyses that the Ovarian Cancer Diagnostics Market is expected to grow at a CAGR of 7.0% in the forecast period of 2023 to 2030 and is expected to reach USD 11,647.12 million by 2030 from USD 6,778.73 million in 2022. There have been a number of new and innovative diagnostic technologies developed for ovarian cancer in recent years. These technologies, such as liquid biopsies and immunohistochemistry, are more accurate and sensitive than traditional diagnostic methods. There is a growing demand for personalized medicine in the healthcare industry. This is leading to the development of new diagnostic tests that can help to identify the best treatment options for individual patients.
Growing incidence and mortality rates of ovarian cancer is expected to drive the market's growth rate
The incidence of ovarian cancer has been increasing in recent years, and this trend is expected to continue. This is due to a number of factors, including the aging population, increased obesity rates, and changes in lifestyle habits. There is a growing awareness about the early signs and symptoms of ovarian cancer, such as abdominal bloating, pelvic pain, and difficulty eating. This awareness is leading to more women seeking early detection tests, such as CA-125 blood tests and transvaginal ultrasounds.
Report Scope and Market Segmentation
|
Report Metric
|
Details
|
|
Forecast Period
|
2023 to 2030
|
|
Base Year
|
2022
|
|
Historic Years
|
2021 (Customizable to 2015- 2020)
|
|
Quantitative Units
|
Revenue in USD Million, Volumes in Units, Pricing in USD
|
|
Segments Covered
|
Product Type (Instruments, Kits and Reagents), Procedure Type (Biopsy Test, Medical Imaging Test, Blood Markers Testing and Genetic Testing), Cancer Type (Germ Cell, Epithelial Tumor and Stromal Cell Tumor), End User (Cancer Diagnostic Centers, Hospital Laboratories, Research Institutes and Others)
|
|
Countries Covered
|
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America
|
|
Market Players Covered
|
Abbott (U.S.), F. Hoffmann-La Roche Ltd (Switzerland), QIAGEN (Germany), Quest Diagnostics Incorporated. (U.S.), Hologic, Inc. (U.S.), Arbor Vita Corporation (U.S.), Guided Therapeutics, Inc. (U.S.), CooperSurgical Inc (U.S.), BD (U.S.), Cardinal Health (U.S.), Siemens Healthcare Private Limited (Germany), Zilico (U.K.), GenomeMe Lab Inc. (Canada), Exact Sciences Corporation (U.S.), Fujirebio (Japan), Advaxis Inc. (U.S.), Pfizer Inc. (U.S.), GlaxoSmithKline plc. (U.K.), Bristol-Myers Squibb Company (U.S.), Merck & Co., Inc (U.S.)
|
|
Data Points Covered in the Report
|
In addition to the market insights such as market value, growth rate, market segments, geographical coverage, market players, and market scenario, the market report curated by the Data Bridge Market Research team includes in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework
|
Segment Analysis:
Global ovarian cancer diagnostics market is categorized into four notable segments based on product type, procedure type, cancer type, and end user.
- Based on product type, the market is segmented into instruments, kits and reagents.
Instruments segment is expected to dominate the ovarian cancer diagnostics market
Instruments segment is expected to dominate the market with 70.27% market share as this type of product is utilized in various regions.
- Based on procedure type, the market is segmented into biopsy tests, medical imaging test, blood markers testing and genetic testing.
Blood markers testing segment is expected to dominate the ovarian cancer diagnostics market
Blood markers testing segment is expected to dominate the market with 40.41% market share as this type of procedure is utilized in various regions.
- Based on cancer type, the market is segmented into germ cell, epithelial tumor, and stromal cell tumor. Epithelial tumor segment is expected to dominate the market with 58.70% market share as this type of cancer is highly prevalent.
- Based on end user, the market is segmented into cancer diagnostic centers, hospital laboratories, research institutes, and others. Cancer diagnostic centers segment is expected to dominate the market with 46.83% market share due to the accurate results and quick detection, which is highly preferred by the manufacturers.
Major Players
Data Bridge Market Research recognizes the following companies as the major market players: Abbott (U.S.), F. Hoffmann-La Roche Ltd (Switzerland), QIAGEN (Germany), Quest Diagnostics Incorporated. (U.S.), Hologic, Inc. (U.S.), Arbor Vita Corporation (U.S.), Guided Therapeutics, Inc. (U.S.), CooperSurgical Inc (U.S.), BD (U.S.), Cardinal Health (U.S.), Siemens Healthcare Private Limited (Germany), Zilico (U.K.), GenomeMe Lab Inc. (Canada), Exact Sciences Corporation (U.S.), Fujirebio (Japan), Advaxis Inc. (U.S.), Pfizer Inc. (U.S.), GlaxoSmithKline plc. (U.K.), Bristol-Myers Squibb Company (U.S.), Merck & Co., Inc (U.S.).
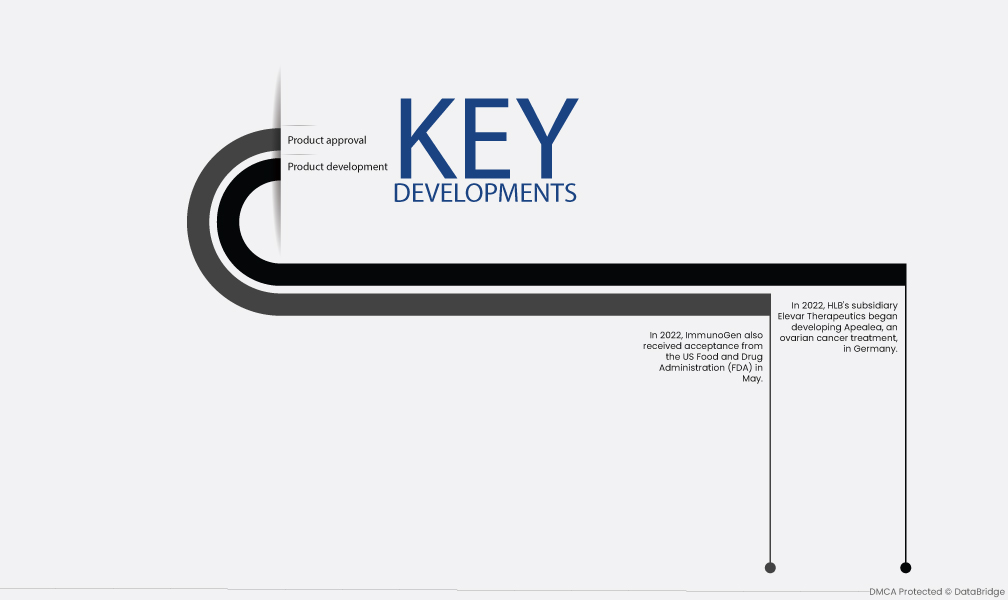
Market Development
- In 2022, HLB's subsidiary Elevar Therapeutics began developing Apealea, an ovarian cancer treatment, in Germany. The drug was listed on Gelbe Liste, a German drug distribution website, and is now available to patients in the country. Apealea is a monoclonal antibody that targets folate receptor alpha, a protein that is overexpressed on the surface of cancer cells. The drug works by binding to folate receptor alpha and delivering a payload of chemotherapy drugs directly to the cancer cells. This helps to kill the cancer cells while minimizing damage to healthy cells.
- In 2022, ImmunoGen also received acceptance from the US Food and Drug Administration (FDA) in May. The organization filed a Biologics License Application for mirvetuximab soravtansine monotherapy, which is a treatment for patients with folate receptor alpha high platinum-resistant ovarian cancer who have been previously treated with 1-3 prior systemic treatments. The FDA is currently reviewing the application. Mirvetuximab soravtansine is a targeted therapy that also works by targeting folate receptor alpha. The drug is a conjugate of a monoclonal antibody and a chemotherapy drug. The monoclonal antibody binds to folate receptor alpha on the surface of cancer cells, and the chemotherapy drug is then released and kills the cancer cells.
Regional Analysis
Geographically, the countries covered in the market report are U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
As per Data Bridge Market Research analysis:
North America is the dominant region in the ovarian cancer diagnostics market during the forecast period 2023-2030
North America ovarian cancer diagnostics market is expected to dominate the global market during the forecast period. The major factors driving the growth of the ovarian cancer diagnostics market in North America are the increasing prevalence of ovarian cancer, technological advancements in the diagnostic procedures, and rising awareness about ovarian cancer.
Asia-Pacific is estimated to be the fastest growing region in the ovarian cancer diagnostics market in the forecast period 2023-2030
Asia-Pacific ovarian cancer diagnostics market is expected to be the fastest-growing market during the forecast period. The major factors driving the growth of the ovarian cancer diagnostics market in Asia-Pacific are the rising awareness about ovarian cancer, increasing government initiatives, and technological advancements in the diagnostic procedures.
For more detailed information about the ovarian cancer diagnostics market report, click here – https://www.databridgemarketresearch.com/reports/global-ovarian-cancer-diagnostics-market