Le succès de la PDTx dépend de son acceptation par les prestataires, qui dépend elle-même de la volonté des organismes payeurs de rémunérer équitablement les médecins pour les médicaments numériques. Selon une enquête de Pear Therapeutics et Avalere, seuls 40 % des organismes payeurs prévoyaient de couvrir la PDTx en 2021, et 50 % d'entre eux prévoyaient de le faire dans les 18 prochains mois. Le principal facteur serait l'efficacité clinique de la PDTx pour améliorer les résultats des patients. Compte tenu de la récente vague d'approbations réglementaires et de la FDA pour la PDTx et de la volonté de la majorité des organismes payeurs de financer les remboursements de la PDTx à l'avenir, les producteurs de PDTx sont rassurés de continuer à innover.
Consultez le rapport complet à l'adresse https://www.databridgemarketresearch.com/reports/global-prescription-digital-therapeutics-dtx-market
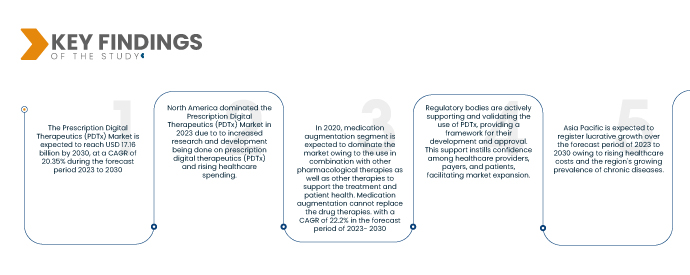
Selon Data Bridge Market Research, le marché des thérapies numériques sur ordonnance (PDTx) représenterait 3,90 milliards USD en 2022 et devrait atteindre 17,16 milliards USD d'ici 2030, avec un TCAC de 20,35 % sur la période de prévision 2023-2030. L'acceptation et l'adoption croissantes des solutions numériques dans le secteur de la santé, ainsi que la reconnaissance de leur efficacité, stimulent la croissance du marché des PDTx. Les professionnels de santé et les patients sont de plus en plus ouverts à l'intégration des thérapies numériques dans leurs plans de traitement.
Principales conclusions de l'étude
La rentabilité et l’accessibilité devraient stimuler le taux de croissance du marché
Les solutions thérapeutiques numériques sur ordonnance (PDTx) offrent une alternative économique aux thérapies traditionnelles, permettant de réduire les dépenses de santé. Grâce aux plateformes numériques, la PDTx élimine le recours à des ressources physiques, telles que les médicaments ou les consultations en personne, réduisant ainsi les coûts associés. De plus, la PDTx améliore l'accessibilité en proposant des options de traitement à distance et autogérées, en éliminant les barrières géographiques et en permettant aux patients d'accéder facilement aux traitements. Cette rentabilité et cette accessibilité accrue font de la PDTx une option attrayante pour les professionnels de santé, les organismes payeurs et les patients à la recherche d'options thérapeutiques efficaces et abordables.
Portée du rapport et segmentation du marché
Rapport métrique
|
Détails
|
Période de prévision
|
2023 à 2030
|
Année de base
|
2022
|
Années historiques
|
2021 (personnalisable de 2015 à 2020)
|
Unités quantitatives
|
Chiffre d'affaires en milliards USD, volumes en unités, prix en USD
|
Segments couverts
|
Mécanisme (mécanismes d'entrée, mécanismes de sortie), catégorie (augmentation de la médication, remplacement de médicament), traitement (traitement ambulatoire, monothérapie), logiciel (logiciel pour les affections respiratoires, logiciel pour la santé mentale, logiciel pour les troubles liés à l'usage d'opioïdes, logiciel pour le diabète, autres), services (microservices comportementaux, microservices médicaux), accessibilité des applications (Android, iOS, Windows), type d'application (applications natives, applications web), application (trouble lié à l'usage de substances (TUS), trouble lié à l'usage d'opioïdes (TOU), trouble déficitaire de l'attention/hyperactivité (TDAH), maladie d'Alzheimer, trouble dépressif majeur (TDM), insomnie, épilepsie, trouble du mouvement, sclérose en plaques, migraine, trouble du spectre autistique, oncologie, inflammation, troubles respiratoires, cardiovasculaires, gestion de la douleur, troubles métaboliques, autres), patients (enfants, adultes)
|
Pays couverts
|
États-Unis, Canada et Mexique en Amérique du Nord, Allemagne, France, Royaume-Uni, Pays-Bas, Suisse, Belgique, Russie, Italie, Espagne, Turquie, Reste de l'Europe en Europe, Chine, Japon, Inde, Corée du Sud, Singapour, Malaisie, Australie, Thaïlande, Indonésie, Philippines, Reste de l'Asie-Pacifique (APAC) en Asie-Pacifique (APAC), Arabie saoudite, Émirats arabes unis, Afrique du Sud, Égypte, Israël, Reste du Moyen-Orient et de l'Afrique (MEA) en tant que partie du Moyen-Orient et de l'Afrique (MEA), Brésil, Argentine et Reste de l'Amérique du Sud en tant que partie de l'Amérique du Sud
|
Acteurs du marché couverts
|
ResMed (États-Unis), SAMSUNGHEALTHCARE (Corée du Sud), Biofourmis (États-Unis), Novartis AG (Suisse), Medtronic (Irlande), Pear Therapeutics, Inc. (États-Unis), Voluntis (France), Omada Health, Inc. (États-Unis), GAIA AG (Allemagne), Welldoc's Bluestar (États-Unis), Solera Network (États-Unis), Akili Interactive Labs, Inc. (États-Unis), Better Therapeutics, LLC (États-Unis), BigHealth (États-Unis), Biofourmis (États-Unis), Click Therapeutics, Inc. (États-Unis), Happify, Inc. (États-Unis), Limbix Health, Inc. (États-Unis), Naturalcycles Nordic AB (Suède), NuvoAir AB (Suède), Sensyne Health plc. (Royaume-Uni), Xealth (États-Unis)
|
Points de données couverts dans le rapport
|
Outre les informations sur les scénarios de marché tels que la valeur marchande, le taux de croissance, la segmentation, la couverture géographique et les principaux acteurs, les rapports de marché organisés par Data Bridge Market Research incluent également une analyse approfondie des experts, une épidémiologie des patients, une analyse du pipeline, une analyse des prix et un cadre réglementaire.
|
Analyse des segments :
Le marché des thérapies numériques sur ordonnance (PDTx) est segmenté sur la base du mécanisme, de la catégorie, du traitement, du logiciel, des services, de l'accessibilité des applications, du type d'application, de l'application et des patients.
- Sur la base de mécanismes, le marché mondial des thérapies numériques sur ordonnance (PDTx) est segmenté en mécanismes d'entrée et mécanismes de sortie. Le segment des mécanismes d'entrée devrait dominer le marché avec un TCAC de 22,2 % sur la période de prévision 2023-2030, en raison de la dépendance des mécanismes aux données des patients soumises via le logiciel ou l'application. Ensuite, l'application ou le logiciel calcule ou suggère l'option thérapeutique aux patients et surveille leurs symptômes.
- Sur la base des segments de catégories, le marché mondial des thérapies numériques sur ordonnance (PDTx) est segmenté en augmentation et substitution médicamenteuses. Le segment des pharmacies hospitalières devrait dominer le marché avec un TCAC de 7,3 % sur la période de prévision 2023-2030, grâce aux thérapies pharmacologiques et autres thérapies qui soutiennent le traitement et la santé des patients.
En 2023, le segment de l'augmentation des médicaments domine le segment de catégorie du marché mondial des thérapies numériques sur ordonnance (PDTx)
En 2023, le segment de l'augmentation médicamenteuse devrait dominer le marché grâce à son utilisation en association avec d'autres thérapies pharmacologiques et d'autres thérapies visant à soutenir le traitement et la santé des patients. L'augmentation médicamenteuse ne peut se substituer aux traitements médicamenteux, avec un TCAC de 22,2 % sur la période de prévision 2023-2030.
- En fonction du segment de traitement, le marché mondial des thérapies numériques sur ordonnance (PDTx) se divise en deux catégories : les traitements ambulatoires et les monothérapies. Le segment des traitements ambulatoires devrait dominer le marché avec un TCAC de 22,2 % sur la période de prévision 2023-2030, car les thérapies numériques sur ordonnance sont des applications ou des logiciels qui fournissent un guide de traitement et des options thérapeutiques adaptées aux symptômes du patient. Les thérapies numériques sur ordonnance sont conçues pour être utilisées à tout moment et en tout lieu, sans consultation médicale.
- En termes de segmentation logicielle, le marché mondial des thérapies numériques sur ordonnance (PDTx) se divise en logiciels pour les affections respiratoires, les problèmes de santé mentale, les troubles liés à l'usage d'opioïdes et le diabète, entre autres. Les logiciels destinés au diabète devraient dominer le marché avec un TCAC de 24,7 % sur la période 2023-2020, en raison de l'augmentation des dépenses mondiales de santé consacrées au diabète et de la recherche continue visant à trouver de nouvelles solutions simples pour traiter les patients.
- Le marché mondial des thérapies numériques sur ordonnance (PDTx) est segmenté en microservices comportementaux et microservices médicaux. Ces derniers devraient dominer le marché avec un TCAC de 22,1 % sur la période 2023-2030, car ces applications logicielles sont principalement axées sur les études comportementales et constituent le principal aspect thérapeutique permettant d'analyser le comportement des patients entre les traitements.
- En fonction de l'accessibilité des applications, le marché mondial des thérapies numériques sur ordonnance (PDTx) est segmenté selon les systèmes Android, iOS et Windows. Ces systèmes sont ensuite subdivisés en smartphones et tablettes. En 2023, le segment des pharmacies hospitalières devrait dominer le marché avec un TCAC de 7,3 % sur la période 2023-2030, en raison des maladies et troubles cardiovasculaires, dont le diabète est la principale cause de décès.
En 2023, le segment Android devrait dominer le segment de l'accessibilité des applications sur le marché mondial des thérapies numériques sur ordonnance (PDTx).
En 2023, le segment Android devrait dominer le marché en raison des maladies et troubles cardiovasculaires, notamment le diabète, première cause de décès. Les organismes de recherche et les acteurs du marché travaillent sans relâche pour traiter les maladies métaboliques qui ont un impact direct sur la vie quotidienne, avec un TCAC de 22,9 % sur la période de prévision 2023-2030.
- Le marché mondial des thérapies numériques sur ordonnance (PDTx) se divise en applications natives et en applications web. Le segment des applications natives devrait dominer le marché avec un TCAC de 22,5 % sur la période de prévision 2023-2030, grâce à l'application personnalisée, exécutée sur un serveur web et stockable localement sur le système d'exploitation.
- Sur la base du segment d'application, le marché mondial des thérapies numériques sur ordonnance (PDTx) est segmenté en troubles liés à l'usage de substances (TUS), troubles liés à l'usage d'opioïdes (TOU), trouble déficitaire de l'attention avec hyperactivité (TDAH), maladie d'Alzheimer, trouble dépressif majeur (TDM), insomnie, épilepsie, troubles du mouvement, sclérose en plaques, migraine, troubles du spectre autistique, oncologie, inflammation, maladies respiratoires, cardiovasculaires, gestion de la douleur, troubles métaboliques, etc. Le segment des maladies métaboliques devrait dominer le marché avec un TCAC de 45,9 % sur la période de prévision de 2023 à 2030, les maladies et troubles cardiovasculaires, en particulier le diabète, étant la principale cause de décès. Les organismes de recherche et les acteurs du marché travaillent en permanence pour traiter les maladies métaboliques qui ont un impact direct sur la vie quotidienne.
- Le marché mondial des thérapies numériques sur ordonnance (PDTx) est segmenté en fonction du segment des patients : enfants et adultes. Le segment adulte devrait dominer le marché avec un TCAC de 22,6 % sur la période de prévision 2023-2030. Les études sur les thérapies numériques sur ordonnance ne portent que sur les adultes, et la majorité de la population adulte utilise ces types de thérapies.
Acteurs majeurs
Data Bridge Market Research reconnaît les entreprises suivantes comme les principaux acteurs du marché des thérapies numériques sur ordonnance (PDTx) : ResMed (États-Unis), SAMSUNGHEALTHCARE (Corée du Sud), Biofourmis (États-Unis), Novartis AG (Suisse), Medtronic (Irlande), Pear Therapeutics, Inc. (États-Unis), Voluntis (France), Omada Health, Inc. (États-Unis), GAIA AG (Allemagne), Welldoc's Bluestar (États-Unis)
Développement du marché
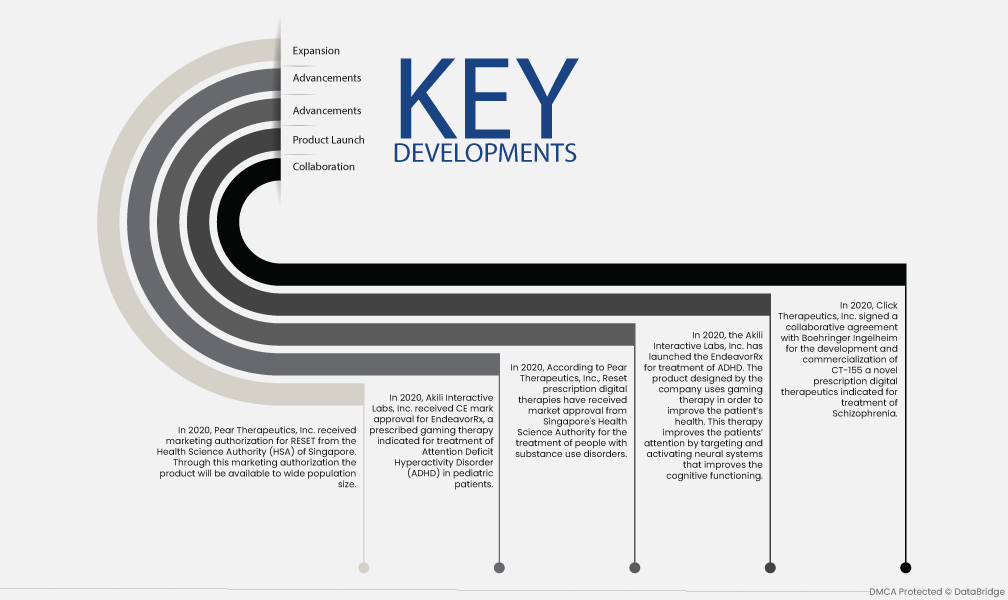
- Expansion - En 2020, Pear Therapeutics, Inc. a reçu l'autorisation de mise sur le marché de RESET de la Health Science Authority (HSA) de Singapour. Grâce à cette autorisation, le produit sera disponible auprès d'un large public.
- Progrès - En 2020, Akili Interactive Labs, Inc. a reçu l'approbation du marquage CE pour EndeavorRx, une thérapie de jeu prescrite indiquée pour le traitement du trouble déficitaire de l'attention avec hyperactivité (TDAH) chez les patients pédiatriques.
- Progrès - En 2020, selon Pear Therapeutics, Inc., les thérapies numériques sur ordonnance Reset ont reçu l'approbation de mise sur le marché de l'Autorité des sciences de la santé de Singapour pour le traitement des personnes souffrant de troubles liés à la consommation de substances.
- Lancement de produit - En 2020, Akili Interactive Labs, Inc. a lancé EndeavorRx pour le traitement du TDAH. Ce produit, conçu par l'entreprise, utilise la thérapie par le jeu pour améliorer la santé du patient. Cette thérapie améliore l'attention en ciblant et en activant les systèmes neuronaux, ce qui améliore les fonctions cognitives.
- Collaboration - En 2020, Click Therapeutics, Inc. a signé un accord de collaboration avec Boehringer Ingelheim pour le développement et la commercialisation du CT-155, une nouvelle thérapie numérique sur ordonnance indiquée pour le traitement de la schizophrénie.
- Collaboration - En 2020, Voluntis a conclu une collaboration avec la société Bristol-Myers Squibb dans le but de créer et d'étudier des thérapies numériques sur ordonnance pour le traitement du cancer.
Analyse régionale
Géographiquement, les pays couverts par le rapport sur le marché des thérapies numériques sur ordonnance (PDTx) sont les États-Unis, le Canada et le Mexique en Amérique du Nord, l'Allemagne, la France, le Royaume-Uni, les Pays-Bas, la Suisse, la Belgique, la Russie, l'Italie, l'Espagne, la Turquie, le reste de l'Europe en Europe, la Chine, le Japon, l'Inde, la Corée du Sud, Singapour, la Malaisie, l'Australie, la Thaïlande, l'Indonésie, les Philippines, le reste de l'Asie-Pacifique (APAC) en Asie-Pacifique (APAC), l'Arabie saoudite, les Émirats arabes unis, l'Afrique du Sud, l'Égypte, Israël, le reste du Moyen-Orient et de l'Afrique (MEA) dans le cadre du Moyen-Orient et de l'Afrique (MEA), le Brésil, l'Argentine et le reste de l'Amérique du Sud dans le cadre de l'Amérique du Sud
Selon l'analyse de Data Bridge Market Research :
L'Amérique du Nord est la région dominante sur le marché des thérapies numériques sur ordonnance (PDTx) au cours de la période de prévision 2023-2030
L'Amérique du Nord occupe une position dominante sur le marché des thérapies numériques sur ordonnance (PDTx) grâce à d'importantes activités de recherche et développement axées sur la PDTx et à des dépenses de santé élevées dans la région. Le marché est également stimulé par l'expansion de grandes entreprises et l'émergence de nouvelles startups, qui favorisent l'innovation et stimulent la croissance. Ces facteurs contribuent à l'accélération de la croissance du marché nord-américain des thérapies numériques sur ordonnance.
L'Asie-Pacifique devrait être la région connaissant la croissance la plus rapide sur le marché des thérapies numériques sur ordonnance (PDTx) au cours de la période de prévision 2023-2030.
La région Asie-Pacifique devrait connaître une croissance significative entre 2023 et 2030, portée par la hausse des coûts de santé et la charge croissante des maladies chroniques. Les dépenses de santé augmentent dans la région, créant une demande pour des solutions rentables comme les thérapies numériques. De plus, l'amélioration des systèmes de paiement des thérapies numériques, notamment les politiques de remboursement et la couverture d'assurance, devrait accélérer l'expansion du marché en Asie-Pacifique, rendant ces traitements innovants plus accessibles aux patients.
Pour plus d'informations sur le marché des thérapies numériques sur ordonnance (PDTx), cliquez ici : https://www.databridgemarketresearch.com/reports/global-prescription-digital-therapeutics-dtx-market