Global Prescription Digital Therapeutics Dtx Market
Market Size in USD Billion
CAGR :
% 
 USD
5.64 Billion
USD
24.86 Billion
2024
2032
USD
5.64 Billion
USD
24.86 Billion
2024
2032
| 2025 –2032 | |
| USD 5.64 Billion | |
| USD 24.86 Billion | |
|
|
|
|
Prescription Digital Therapeutics (PDTx) Market Size
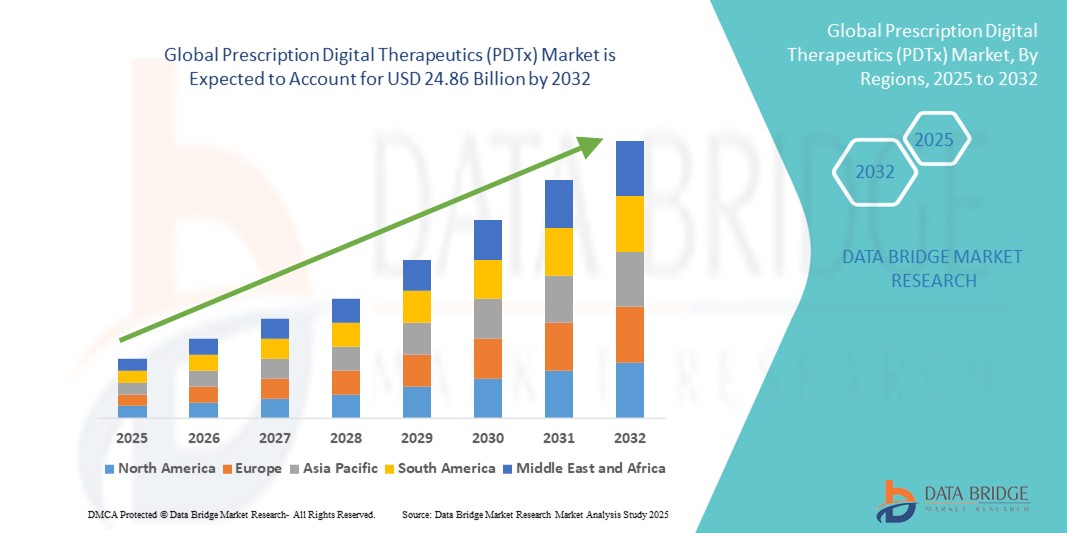
- The global prescription digital therapeutics (PDTx) market size was valued at USD 5.64 billion in 2024 and is expected to reach USD 24.86 billion by 2032, at a CAGR of 20.35% during the forecast period
- The market growth is primarily driven by the increasing prevalence of chronic diseases, mental health disorders, and the need for scalable, evidence-based digital treatments that go beyond traditional care models
- In addition, growing regulatory support, rising adoption of digital health tools by healthcare providers, and patient preference for non-invasive, accessible therapies are positioning PDTx as a transformative component of personalized healthcare. These trends are propelling significant momentum in the PDTx industry, fostering widespread clinical integration and market expansion
Prescription Digital Therapeutics (PDTx) Market Analysis
- Prescription digital therapeutics (PDTx), which deliver clinically validated software-based interventions for treating a variety of medical conditions, are becoming increasingly integral to modern healthcare due to their ability to provide scalable, personalized, and non-invasive therapies through digital platforms
- The growing adoption of PDTx is driven by the rising prevalence of chronic and mental health conditions, increasing demand for remote healthcare solutions, and favorable regulatory support for digital therapeutics integration into traditional care models
- North America dominated the prescription digital therapeutics (PDTx) market with the largest revenue share of 47.2% in 2024, supported by a well-established digital infrastructure, regulatory approvals by agencies such as the FDA, and significant investments by both pharma and tech giants, with the U.S. leading in commercial adoption and clinical deployment of PDTx solutions
- Asia-Pacific is projected to be the fastest growing region in the prescription digital therapeutics (PDTx) market during the forecast period due to rising digital literacy, increased smartphone usage, and expanding telehealth initiatives across countries such as India, China, and Japan
- Software for Mental Health segment dominated the prescription digital therapeutics (PDTx) market with a market share of 42% in 2024, fueled by the escalating burden of anxiety, depression, and stress-related disorders, and the proven clinical efficacy of PDTx interventions in delivering cognitive behavioral therapy digitally
Report Scope and Prescription Digital Therapeutics (PDTx) Market Segmentation
|
Attributes |
Prescription Digital Therapeutics (PDTx) Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Prescription Digital Therapeutics (PDTx) Market Trends
Expansion of AI-Driven Personalization and Integration with Remote Care Platforms
- A defining trend in the global PDTx market is the growing incorporation of artificial intelligence (AI) and machine learning algorithms to personalize therapeutic interventions and enhance treatment efficacy. These advanced capabilities enable PDTx solutions to dynamically adapt to user behavior, health data, and real-time progress, creating a more individualized and effective therapy experience
- For instance, Pear Therapeutics' reSET-O uses adaptive algorithms to support patients with opioid use disorder, adjusting treatment pathways based on real-time engagement. Similarly, apps such as Big Health’s Sleepio utilize AI to tailor cognitive behavioral therapy for insomnia, learning from user inputs and patterns
- Integration with telehealth platforms and electronic health records (EHRs) is also accelerating, enabling seamless data sharing between patients and providers. This allows clinicians to monitor progress, intervene when necessary, and make data-driven decisions about treatment modifications
- The convergence of PDTx with broader digital health ecosystems—such as wearable health trackers and remote monitoring tools supports more holistic, continuous care outside of traditional clinical settings. This interconnected approach is reshaping chronic disease management by providing timely, real-world insights
- Companies such as Akili Interactive and Click Therapeutics are leveraging AI for real-time feedback, engagement tracking, and outcome prediction, setting a new standard for personalized, technology-enabled therapeutic care
- As demand for cost-effective, scalable, and accessible treatment continues to grow, AI-enhanced PDTx solutions are poised to become a foundational component of the future digital health landscape
Prescription Digital Therapeutics (PDTx) Market Dynamics
Driver
Rising Demand for Scalable Mental Health and Chronic Disease Solutions
- The global increase in chronic conditions and mental health disorders, alongside limitations in traditional healthcare infrastructure, is fueling demand for scalable, non-invasive therapeutic solutions such as PDTx
- For instance, in March 2024, the FDA approved Otsuka and Click Therapeutics’ reSET-A, a PDTx solution targeting major depressive disorder (MDD), reinforcing the clinical credibility and therapeutic value of these software-based interventions
- PDTx platforms offer several advantages, including real-time monitoring, standardized interventions, and the ability to reach patients in underserved or remote areas. These features make PDTx an attractive option for healthcare providers and patients asuch as
- In addition, the growing emphasis on value-based care and preventive treatment is pushing health systems and insurers to embrace PDTx as cost-effective alternatives or complements to medication and therapy
- The convenience of smartphone-based delivery, 24/7 accessibility, and progress tracking features enhance patient engagement and adherence critical factors in achieving better outcomes in mental health, diabetes, and substance use treatment
Restraint/Challenge
Regulatory, Reimbursement, and Awareness Gaps
- Despite the promise of PDTx, several barriers continue to slow widespread adoption. These include fragmented regulatory pathways, varying regional definitions of PDTx, and a lack of standardized reimbursement models from payers
- For instance, while the FDA has approved multiple PDTx products under the Software as a Medical Device (SaMD) framework, many regions lack equivalent approval processes, creating inconsistency and market entry challenges for developers
- In addition, limited awareness and understanding of PDTx among both healthcare providers and patients hampers prescribing and usage rates. Unsuch as traditional medications, PDTx often require training and technical familiarity, which can deter adoption
- Companies must also navigate concerns about data privacy, security, and the ethical use of patient-generated health data, especially in markets with strict regulations such as GDPR in Europe
- To overcome these hurdles, stakeholders must work collaboratively to establish clear regulatory guidelines, expand coverage and reimbursement, and implement robust education initiatives that emphasize the safety, efficacy, and value of PDTx in modern care models
Prescription Digital Therapeutics (PDTx) Market Scope
The market is segmented on the basis of mechanism, category, treatment, software, services, app accessibility, app type, application, and patients.
- By Mechanism
On the basis of mechanism, the prescription digital therapeutics (PDTx) market is segmented into input mechanisms and output mechanisms. The input mechanisms segment held the larger market revenue share in 2024, driven by its foundational role in capturing patient-generated health data through mobile devices, sensors, and questionnaires. These inputs are essential for enabling real-time monitoring, dynamic therapy adjustments, and personalized care pathways. The growth of wearable technologies and smartphone adoption further strengthens the dominance of input mechanisms in the PDTx ecosystem.
The output mechanisms segment is expected to witness steady growth during the forecast period, as software platforms increasingly incorporate interactive content, behavioral nudges, and adaptive feedback to drive patient engagement and therapy adherence. These outputs are crucial for delivering therapeutic benefits in mental health, chronic conditions, and cognitive disorders.
- By Category
On the basis of category, the prescription digital therapeutics (PDTx) market is segmented into medication augmentation and medication replacement. The medication augmentation segment dominated the market in 2024, with the largest revenue share, due to its ability to enhance treatment outcomes when used alongside pharmacological interventions. PDTx solutions in this category are increasingly prescribed by clinicians to support behavioral therapies, monitor medication adherence, and deliver education modules, particularly in mental health and substance use disorder treatment.
The medication replacement segment is anticipated to grow at a notable rate, driven by the regulatory approval of standalone PDTx therapies that serve as digital-first treatments, especially in mild to moderate conditions such as insomnia and anxiety. These platforms appeal to users seeking non-pharmacological, low-risk interventions with clinically validated results.
- By Treatment
On the basis of treatment, the prescription digital therapeutics (PDTx) market is segmented into outpatient treatment and monotherapy. The outpatient treatment segment held the majority market share in 2024, supported by its widespread use in chronic care management and behavioral health programs. PDTx platforms enable remote delivery of therapy, eliminating the need for frequent clinic visits and enhancing accessibility for patients across diverse geographies.
The monotherapy segment is gaining traction during forecast period, particularly in conditions such as ADHD, where digital therapeutics are FDA-approved to deliver primary treatment without adjunct medications. Growing clinical evidence and patient preference for non-drug interventions are expected to drive this segment forward.
- By Software
On the basis of software, the prescription digital therapeutics (PDTx) market is segmented by software into software for mental health, software for opioid use disorder, software for diabetes, software for respiratory conditions, and others. The mental health software segment dominated the market in 2024 with the largest revenue share of 42%, fueled by increasing prevalence of depression, anxiety, and stress-related disorders. Clinically validated solutions delivering cognitive behavioral therapy (CBT) digitally are widely adopted by both patients and healthcare providers.
The software for diabetes segment is projected to grow rapidly during forecast period, supported by rising global diabetes incidence and the proven efficacy of PDTx in lifestyle management, blood glucose monitoring, and behavioral coaching.
- By Services
On the basis of services, the prescription digital therapeutics (PDTx) market is divided into behavioral microservices and medical microservices. The behavioral microservices segment held the dominant share in 2024, driven by strong uptake of digital CBT, motivational interviewing tools, and adherence-focused behavioral interventions.
Medical microservices are expected to witness increased adoption during the forecast period, as PDTx expands into conditions such as cardiovascular and metabolic disorders, requiring clinical-grade data capture and integration with electronic health records.
- By App Accessibility
On the basis of app accessibility, the prescription digital therapeutics (PDTx) market is segmented into Android, iOS, and Windows. The Android segment held the largest market share in 2024, driven by the platform’s global smartphone dominance and ease of access in emerging markets. PDTx developers often prioritize Android for initial rollouts due to its broader user base and compatibility with budget devices.
The iOS segment is expected to witness fastest growth during forecast period, as it commands strong presence in high-income regions and is preferred for premium PDTx offerings, especially in North America and Western Europe.
- By App Type
On the basis of app type, the prescription digital therapeutics (PDTx) market is segmented into native apps and web apps based on app type. Native apps dominated the PDTx market in 2024 due to their superior performance, offline access, and advanced features such as push notifications, biometric security, and real-time data syncing. These characteristics make native apps ideal for delivering personalized and interactive therapeutic content.
Web apps, is expected to witness fastest growth during forecast period, as it offer cross-platform accessibility and faster updates, and as developers seek scalable, browser-based solutions for broader reach.
- By Application
On the basis of application segment, the prescription digital therapeutics (PDTx) market is segmented into substance use disorder (SUD), opioid use disorder (OUD), attention deficit/hyperactivity disorder (ADHD), Alzheimer’s disease, major depressive disorder (MDD), insomnia, epilepsy, movement disorder, multiple sclerosis, migraine, autism spectrum disorder, oncology, inflammation, respiratory, cardiovascular, pain management, metabolic conditions, and others. The major depressive disorder (MDD) segment held the largest market share in 2024, driven by increasing mental health awareness and the availability of FDA-approved PDTx solutions such as reSET-A and Sleepio.
The Attention Deficit/Hyperactivity Disorder (ADHD) segment is experiencing rapid growth during forecast period, with digital therapeutics such as EndeavorRx and Somryst gaining recognition for their clinical efficacy and patient-centered design.
- By Patients
On the basis of patient group, the prescription digital therapeutics (PDTx) market is segmented into children and adults. The adult segment dominated the market in 2024 due to the high prevalence of chronic and behavioral health conditions among the adult population. Adults are also more such asly to own smartphones and engage with mobile health solutions, supporting higher adoption rates for PDTx.
The children segment is projected to expand during forecast period, with the growing use of PDTx for pediatric conditions such as ADHD and autism, supported by increasing regulatory approvals and parental demand for safe, digital-first interventions.
Prescription Digital Therapeutics (PDTx) Market Regional Analysis
- North America dominated the prescription digital therapeutics (PDTx) market with the largest revenue share of 47.2% in 2024, supported by a well-established digital infrastructure, regulatory approvals by agencies such as the FDA, and significant investments by both pharma and tech giants, with the U.S. leading in commercial adoption and clinical deployment of PDTx solutions
- The region's strong digital infrastructure, increasing acceptance of app-based therapy, and rising partnerships between tech firms and healthcare providers fuel widespread implementation of PDTx solutions
- In addition, the presence of leading PDTx developers, coupled with FDA approvals and reimbursement support, positions North America as a mature and innovation-driven market, especially for indications such as MDD, ADHD, and substance use disorders
U.S. Prescription Digital Therapeutics (PDTx) Market Insight
The U.S. prescription digital therapeutics (PDTx) market accounted for the largest revenue share of 82.6% in North America in 2024, driven by high digital literacy, early regulatory approvals by the FDA, and increasing payer reimbursement for PDTx solutions. The strong presence of key players, including Pear Therapeutics and Akili Interactive, and the focus on conditions such as depression, ADHD, and substance use disorders, are driving adoption. Integration with electronic health records (EHRs) and growing clinical validation of app-based interventions continue to support market expansion across outpatient settings.
Europe Prescription Digital Therapeutics (PDTx) Market Insight
The Europe prescription digital therapeutics (PDTx) market is projected to grow at a significant CAGR throughout the forecast period, spurred by rising healthcare digitization initiatives and supportive regulatory frameworks such as the EU Medical Device Regulation (MDR). Increasing awareness among healthcare providers and patients about non-invasive, software-based treatments is promoting widespread adoption. Chronic disease burden, along with favorable health insurance policies and investments in digital health infrastructure, is boosting the use of PDTx for mental health and metabolic conditions across both public and private health systems.
U.K. Prescription Digital Therapeutics (PDTx) Market Insight
The U.K. prescription digital therapeutics (PDTx) market is expected to witness robust growth during the forecast period, supported by the National Health Service’s (NHS) digital-first strategy and growing mental health challenges post-COVID. The country's progressive stance on digital health and increased investments in digital therapeutics platforms are accelerating adoption. The focus on reducing treatment gaps in behavioral health and chronic disease management positions the U.K. as a critical market in Europe for validated PDTx interventions.
Germany Prescription Digital Therapeutics (PDTx) Market Insight
The Germany prescription digital therapeutics (PDTx) market is poised for substantial growth, driven by the pioneering DiGA Fast Track framework, which allows digital health apps to be prescribed and reimbursed. With a strong emphasis on regulatory compliance and evidence-based efficacy, Germany has created a favorable environment for PDTx adoption. The rising incidence of lifestyle-related disorders and mental health conditions, alongside increasing patient engagement in self-care, is reinforcing the demand for prescription-based therapeutic software in both inpatient and outpatient care.
Asia-Pacific Prescription Digital Therapeutics (PDTx) Market Insight
The Asia-Pacific prescription digital therapeutics (PDTx) market is forecasted to grow at the fastest CAGR of 25.7% from 2025 to 2032, fueled by digital health transformation efforts in emerging economies. Rapid urbanization, expanding internet penetration, and increasing investments in healthcare IT infrastructure are driving the market. Government-backed digital health policies in countries such as India, China, and Japan, coupled with a rising need for scalable mental health and chronic disease solutions, are enhancing the adoption of PDTx across hospitals and clinics.
Japan Prescription Digital Therapeutics (PDTx) Market Insight
The Japan prescription digital therapeutics (PDTx) market is gaining traction due to its aging population, rising chronic disease burden, and government efforts to digitize healthcare under the “Society 5.0” vision. High smartphone penetration, patient openness to digital tools, and insurance initiatives supporting digital health solutions make Japan a fertile ground for PDTx deployment. Mental health and cognitive decline in the elderly are key therapeutic areas being addressed through software-based interventions integrated with clinical workflows.
India Prescription Digital Therapeutics (PDTx) Market Insight
The India prescription digital therapeutics (PDTx) market captured the largest revenue share in the Asia-Pacific PDTx market in 2024, driven by its fast-growing digital health ecosystem, increased smartphone adoption, and government-backed programs such as Ayushman Bharat Digital Mission. The rising prevalence of diabetes, hypertension, and depression is prompting demand for accessible, low-cost therapeutic solutions. The country's large patient pool, digital-savvy youth population, and a growing base of health-tech startups are positioning India as a vital market for scalable PDTx solutions.
Prescription Digital Therapeutics (PDTx) Market Share
The prescription digital therapeutics (PDTx) industry is primarily led by well-established companies, including:
- ResMed (U.S.)
- SAMSUNGHEALTHCARE (South Korea)
- Biofourmis (US)
- Novartis AG (Switzerland)
- Medtronic (Ireland)
- Pear Therapeutics, Inc. (U.S.)
- Voluntis (France)
- Omada Health, Inc. (U.S.)
- GAIA AG (Germany)
- Welldoc’s Bluestar (U.S.)
- Solera Network (U.S.)
- Akili Interactive Labs, Inc. (U.S.)
- Better Therapeutics, LLC (U.S.)
- BigHealth (U.S.)
- Biofourmis (U.S.)
- Click Therapeutics, Inc. (U.S.)
- Happify, Inc. (U.S.)
- Limbix Health, Inc. (U.S.)
- Naturalcycles Nordic AB (Sweden)
- NuvoAir AB (Sweden)
- Sensyne Health plc. (U.K.)
- Xealth (U.S.)
What are the Recent Developments in Global Prescription Digital Therapeutics (PDTx) Market?
- In May 2025, U.S. lawmakers reintroduced the Access to Prescription Digital Therapeutics Act, seeking to establish Medicare and Medicaid reimbursement pathways for FDA-authorized PDTx. The bill represents a pivotal step toward formal payer recognition of digital therapeutics as legitimate, reimbursable treatments and is expected to enhance adoption, affordability, and provider engagement across the U.S. healthcare system
- In May 2025, the Centers for Medicare & Medicaid Services (CMS), together with the Office of the National Coordinator for Health Information Technology (ONC), issued a Request for Information (RFI) titled “Health Technology Ecosystem.” The aim is to gather public input on the digital health landscape for Medicare beneficiaries, focusing on data interoperability and broader health technology infrastructure
- In April 2025, Click Therapeutics secured FDA marketing authorization for CT‑132, the first prescription digital therapeutic approved for the preventive treatment of episodic migraine in adults under the De Novo classification. This groundbreaking approval underscores the expanding clinical relevance of PDTx solutions beyond behavioral and mental health, highlighting regulatory confidence in digital platforms as viable and effective treatment modalities. The development marks a major milestone in the evolution of digital health and positions Click Therapeutics as a leading innovator in neurology-focused PDTx
- In March 2025, Dassault Systèmes expanded its strategic alliance with Click Therapeutics by investing directly in the company to support advanced digital therapeutics development. This move aims to connect clinical research and digital patient engagement via Dassault’s Medidata platform, thereby strengthening the pipeline of software-driven treatments from clinical trials to market delivery. This partnership reflects a broader trend of traditional life sciences firms integrating AI and software to redefine therapeutic value across the continuum of care
- In June 2024, Akili Interactive received FDA clearance for EndeavorOTC, the first over-the-counter digital therapeutic for adult ADHD, allowing broader public access without a physician's prescription. Previously available as a pediatric prescription therapy, this version represents a major leap in democratizing digital care access and making neurocognitive therapy more scalable. The milestone sets a precedent for future non-prescription PDTx solutions to enter consumer markets
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL PRESCRIPTION DIGITAL THERAPEUTICS (PDTX) MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE PRESCRIPTION DIGITAL THERAPEUTICS (PDTX) SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 SALES VOLUME DATA
2.2.12 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.13 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 GLOBAL PRESCRIPTION DIGITAL THERAPEUTICS (PDTX) MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 MARKET OVERVIEW
3.1 DRIVERS
3.2 RESTRAINTS
3.3 OPPORTUNITIES
3.4 CHALLENGES
4 EXECUTIVE SUMMARY
5 PREMIUM INSIGHTS
5.1 PESTEL ANALYSIS
5.2 PORTER’S FIVE FORCES MODEL
6 INDUSTRY INSIGHTS
6.1 MICRO AND MACRO ECONOMIC FACTORS
6.2 PENETRATION AND GROWTH PROSPECT MAPPING
6.3 KEY PRICING STRATEGIES
6.4 INTERVIEWS WITH SPECIALIST
6.5 ANALYIS AND RECOMMENDATION
7 INTELLECTUAL PROPERTY (IP) PORTFOLIO
7.1 PATENT QUALITY AND STRENGTH
7.2 PATENT FAMILIES
7.3 LICENSING AND COLLABORATIONS
7.4 COMPETITIVE LANDSCAPE
7.5 IP STRATEGY AND MANAGEMENT
7.6 OTHER
8 COST ANALYSIS BREAKDOWN
9 TECHNONLOGY ROADMAP
10 INNOVATION TRACKER AND STRATEGIC ANALYSIS
10.1 MAJOR DEALS AND STRATEGIC ALLIANCES ANALYSIS
10.1.1 JOINT VENTURES
10.1.2 MERGERS AND ACQUISITIONS
10.1.3 LICENSING AND PARTNERSHIP
10.1.4 TECHNOLOGY COLLABORATIONS
10.1.5 STRATEGIC DIVESTMENTS
10.2 NUMBER OF PRODUCTS IN DEVELOPMENT
10.3 STAGE OF DEVELOPMENT
10.4 TIMELINES AND MILESTONES
10.5 INNOVATION STRATEGIES AND METHODOLOGIES
10.6 RISK ASSESSMENT AND MITIGATION
10.7 MERGERS AND ACQUISITIONS
10.8 FUTURE OUTLOOK
11 REGULATORY COMPLIANCE
11.1 REGULATORY AUTHORITIES
11.2 REGULATORY CLASSIFICATIONS
11.3 REGULATORY SUBMISSIONS
11.4 INTERNATIONAL HARMONIZATION
11.5 COMPLIANCE AND QUALITY MANAGEMENT SYSTEMS
11.6 REGULATORY CHALLENGES AND STRATEGIES
FIGURE 1 TOP ENTITIES BASED ON R&D GLANCE FOR GLOBAL PRESCRIPTION DIGITAL THERAPEUTICS (PDTX) MARKET
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
12 REIMBURSEMENT FRAMEWORK
13 OPPUTUNITY MAP ANALYSIS
14 VALUE CHAIN ANALYSIS
15 HEALTHCARE ECONOMY
15.1 HEALTHCARE EXPENDITURE
15.2 CAPITAL EXPENDITURE
15.3 CAPEX TRENDS
15.4 CAPEX ALLOCATION
15.5 FUNDING SOURCES
15.6 INDUSTRY BENCHMARKS
15.7 GDP RATION IN OVERALL GDP
15.8 HEALTHCARE SYSTEM STRUCTURE
15.9 GOVERNMENT POLICIES
15.1 ECONOMIC DEVELOPMENT
16 GLOBAL PRESCRIPTION DIGITAL THERAPEUTICS (DTX) MARKET, BY MECHANISM
16.1 OVERVIEW
16.2 INPUT MECHANISMS
16.2.1 BY TYPE
16.2.1.1. SCREEN
16.2.1.2. ACCELEROMETER
16.2.1.3. MICROPHONE
16.2.2 BY APPLICATION
16.2.2.1. PATIENT DATA COLLECTION
16.2.2.1.1. MOOD
16.2.2.1.2. COGNITION
16.2.2.1.3. MEMORY
16.2.2.1.4. FAMILIAL RECOGNITION
16.2.2.1.5. CONCENTRATION
16.2.2.1.6. OTHERS
16.2.2.2. DEXTERITY OR STEADINESS MEASUREMENT
16.2.2.2.1. STRETCHING
16.2.2.2.2. RANGE OF MOTION TESTS
16.2.2.2.3. OTHERS
16.2.2.3. AUDIO ANALYSIS
16.2.2.3.1. VOICE QUALITY
16.2.2.3.2. SHAKINESS
16.2.2.3.3. OTHERS
16.3 OUTPUT MECHANISMS
16.3.1 BY TYPE
16.3.1.1. SCREEN
16.3.1.2. CHAT
16.3.1.3. NOTIFICATION
16.3.2 BY APPLICATION
16.3.2.1. CONTENT DELIVERY
16.3.2.1.1. BLOGS
16.3.2.1.2. TIPS
16.3.2.1.3. FAQS
16.3.2.1.4. VIDEOS
16.3.2.1.5. OTHERS
16.3.2.2. PATIENT COMMUNICATION
16.3.2.2.1. PROFESSIONAL COACHING
16.3.2.2.2. PEER-TO-PEER SUPPORT
16.3.2.2.3. OTHERS
16.3.2.3. ADHERENCE PROMPTS
16.3.2.3.1. INACTIVITY
16.3.2.3.2. SLEEP
16.3.2.3.3. DIET
16.3.2.3.4. OTHERS
17 GLOBAL PRESCRIPTION DIGITAL THERAPEUTICS (DTX) MARKET, BY TREATMENT
17.1 OVERVIEW
17.2 BY USAGE
17.2.1 MEDICATION AUGMENTATION
17.2.2 MEDICATION REPLACEMENT
17.3 BY TYPE
17.3.1 OUTPATIENT TREATMENT
17.3.1.1. COGNITIVE BEHAVIORAL THERAPY (CBT)
17.3.1.2. FLUENCY TRANING
17.3.1.3. CONTIGENCY MANAGEMENT
17.3.1.4. CRAVING AND TRIGGER ASSESSMENT
17.3.1.5. TRANSMUCOSAL BUPRENORPHINE
17.3.1.6. OTHERS
17.3.2 MONOTHERAPY
17.3.2.1. INSOMNIA-SPECIFIC COGNITIVE BEHAVIORAL THERAPY (CBTI)
17.3.2.2. COGNITIVE RESTRUCTURING
17.3.2.3. OTHERS
17.3.3 OTHERS
18 GLOBAL PRESCRIPTION DIGITAL THERAPEUTICS (DTX) MARKET, BY SOFTWARE
18.1 OVERVIEW
18.2 SOFTWARE FOR RESPIATIORY CONDITIONS
18.3 SOFTWARE FOR MENTAL HEALTH
18.4 SOFTWARE FOR OPIOID USE DISORDER
18.5 SOFTWARE FOR DIABETES
18.6 OTHERS
19 GLOBAL PRESCRIPTION DIGITAL THERAPEUTICS (DTX) MARKET, BY SERVICES
19.1 OVERVIEW
19.2 BEHAVIORAL MICROSERVICES
19.2.1 PHYSICAL ACITIVITY
19.2.2 RELAXATION TECHNIQUE
19.2.3 DIET OPTIMIZATION
19.2.4 CONTROLLED BREATHING
19.2.5 TELEMEDICINE
19.2.6 MINDFULNESS TRAINING
19.2.7 OTHERS
19.3 MEDICAL MICROSERVICES
19.3.1 MEDICATION MANAGAMENT SYSTEM
19.3.2 FAGERSTROM TEST
19.3.3 PATIENT HEALTH QUESTIONNAIRE
19.3.4 FINANCIAL SAVING ENGINE
19.3.5 HEALTH RECOVERED ENGINE
19.3.6 MORISKY MEDICATION ADHERENCE SCALE
19.3.7 OTHERS
20 GLOBAL PRESCRIPTION DIGITAL THERAPEUTICS (DTX) MARKET, BY APP ACCESSIBILITY
20.1 OVERVIEW
20.2 ANDROID
20.2.1 SMARTPHONE
20.2.2 TABLET
20.3 IOS
20.3.1 SMARTPHONE
20.3.2 TABLET
20.4 WINDOWS
20.4.1 SMARTPHONE
20.4.2 TABLET
21 GLOBAL PRESCRIPTION DIGITAL THERAPEUTICS (DTX) MARKET, BY APP TYPE
21.1 OVERVIEW
21.2 NATIVE APPS
21.3 WEB APPS
21.4 HYBRID APPS
22 GLOBAL PRESCRIPTION DIGITAL THERAPEUTICS (DTX) MARKET, BY APPLICATION
22.1 OVERVIEW
22.2 SUBSTANCE USE DISORDER (SUD)
22.2.1 BY USAGE
22.2.1.1. MEDICATION AUGMENTATION
22.2.1.2. MEDICATION REPLACEMENT
22.2.2 BY TYPE
22.2.2.1. OUTPATIENT TREATMENT
22.2.2.1.1. COGNITIVE BEHAVIORAL THERAPY (CBT)
22.2.2.1.2. FLUENCY TRANING
22.2.2.1.3. CONTIGENCY MANAGEMENT
22.2.2.1.4. CRAVING AND TRIGGER ASSESSMENT
22.2.2.1.5. TRANSMUCOSAL BUPRENORPHINE
22.2.2.1.6. OTHERS
22.2.2.2. MONOTHERAPY
22.2.2.2.1. INSOMNIA-SPECIFIC COGNITIVE BEHAVIORAL THERAPY (CBTI)
22.2.2.2.2. COGNITIVE RESTRUCTURING
22.2.2.2.3. OTHERS
22.2.2.3. OTHERS
22.3 OPIOID USE DISORDER (OUD)
22.3.1 BY USAGE
22.3.1.1. MEDICATION AUGMENTATION
22.3.1.2. MEDICATION REPLACEMENT
22.3.2 BY TYPE
22.3.2.1. OUTPATIENT TREATMENT
22.3.2.1.1. COGNITIVE BEHAVIORAL THERAPY (CBT)
22.3.2.1.2. FLUENCY TRANING
22.3.2.1.3. CONTIGENCY MANAGEMENT
22.3.2.1.4. CRAVING AND TRIGGER ASSESSMENT
22.3.2.1.5. TRANSMUCOSAL BUPRENORPHINE
22.3.2.1.6. OTHERS
22.3.2.2. MONOTHERAPY
22.3.2.2.1. INSOMNIA-SPECIFIC COGNITIVE BEHAVIORAL THERAPY (CBTI)
22.3.2.2.2. COGNITIVE RESTRUCTURING
22.3.2.2.3. OTHERS
22.3.2.3. OTHERS
22.4 ATTENTION DEFICIT/HYPERACTIVITY DISORDER (ADHD)
22.4.1 BY USAGE
22.4.1.1. MEDICATION AUGMENTATION
22.4.1.2. MEDICATION REPLACEMENT
22.4.2 BY TYPE
22.4.2.1. OUTPATIENT TREATMENT
22.4.2.1.1. COGNITIVE BEHAVIORAL THERAPY (CBT)
22.4.2.1.2. FLUENCY TRANING
22.4.2.1.3. CONTIGENCY MANAGEMENT
22.4.2.1.4. CRAVING AND TRIGGER ASSESSMENT
22.4.2.1.5. TRANSMUCOSAL BUPRENORPHINE
22.4.2.1.6. OTHERS
22.4.2.2. MONOTHERAPY
22.4.2.2.1. INSOMNIA-SPECIFIC COGNITIVE BEHAVIORAL THERAPY (CBTI)
22.4.2.2.2. COGNITIVE RESTRUCTURING
22.4.2.2.3. OTHERS
22.5 OTHERSALZHEIMER’S DISEASE
22.5.1 BY USAGE
22.5.1.1. MEDICATION AUGMENTATION
22.5.1.2. MEDICATION REPLACEMENT
22.5.2 BY TYPE
22.5.2.1. OUTPATIENT TREATMENT
22.5.2.1.1. COGNITIVE BEHAVIORAL THERAPY (CBT)
22.5.2.1.2. FLUENCY TRANING
22.5.2.1.3. CONTIGENCY MANAGEMENT
22.5.2.1.4. CRAVING AND TRIGGER ASSESSMENT
22.5.2.1.5. TRANSMUCOSAL BUPRENORPHINE
22.5.2.1.6. OTHERS
22.5.2.2. MONOTHERAPY
22.5.2.2.1. INSOMNIA-SPECIFIC COGNITIVE BEHAVIORAL THERAPY (CBTI)
22.5.2.2.2. COGNITIVE RESTRUCTURING
22.5.2.2.3. OTHERS
22.5.2.3. OTHERS
22.6 MAJOR DEPRESSIVE DISORDER (MDD)
22.6.1 BY USAGE
22.6.1.1. MEDICATION AUGMENTATION
22.6.1.2. MEDICATION REPLACEMENT
22.6.2 BY TYPE
22.6.2.1. OUTPATIENT TREATMENT
22.6.2.1.1. COGNITIVE BEHAVIORAL THERAPY (CBT)
22.6.2.1.2. FLUENCY TRANING
22.6.2.1.3. CONTIGENCY MANAGEMENT
22.6.2.1.4. CRAVING AND TRIGGER ASSESSMENT
22.6.2.1.5. TRANSMUCOSAL BUPRENORPHINE
22.6.2.1.6. OTHERS
22.6.2.2. MONOTHERAPY
22.6.2.2.1. INSOMNIA-SPECIFIC COGNITIVE BEHAVIORAL THERAPY (CBTI)
22.6.2.2.2. COGNITIVE RESTRUCTURING
22.6.2.2.3. OTHERS
22.6.2.3. OTHERS
22.7 INSOMNIA
22.7.1 BY USAGE
22.7.1.1. MEDICATION AUGMENTATION
22.7.1.2. MEDICATION REPLACEMENT
22.7.2 BY TYPE
22.7.2.1. OUTPATIENT TREATMENT
22.7.2.1.1. COGNITIVE BEHAVIORAL THERAPY (CBT)
22.7.2.1.2. FLUENCY TRANING
22.7.2.1.3. CONTIGENCY MANAGEMENT
22.7.2.1.4. CRAVING AND TRIGGER ASSESSMENT
22.7.2.1.5. TRANSMUCOSAL BUPRENORPHINE
22.7.2.1.6. OTHERS
22.7.2.2. MONOTHERAPY
22.7.2.2.1. INSOMNIA-SPECIFIC COGNITIVE BEHAVIORAL THERAPY (CBTI)
22.7.2.2.2. COGNITIVE RESTRUCTURING
22.7.2.2.3. OTHERS
22.7.2.3. OTHERS
22.8 COGNITION
22.8.1 BY USAGE
22.8.1.1. MEDICATION AUGMENTATION
22.8.1.2. MEDICATION REPLACEMENT
22.8.2 BY TYPE
22.8.2.1. OUTPATIENT TREATMENT
22.8.2.1.1. COGNITIVE BEHAVIORAL THERAPY (CBT)
22.8.2.1.2. FLUENCY TRANING
22.8.2.1.3. CONTIGENCY MANAGEMENT
22.8.2.1.4. CRAVING AND TRIGGER ASSESSMENT
22.8.2.1.5. TRANSMUCOSAL BUPRENORPHINE
22.8.2.1.6. OTHERS
22.8.2.2. MONOTHERAPY
22.8.2.2.1. INSOMNIA-SPECIFIC COGNITIVE BEHAVIORAL THERAPY (CBTI)
22.8.2.2.2. COGNITIVE RESTRUCTURING
22.8.2.2.3. OTHERS
22.8.2.3. OTHERS
22.9 EPILEPSY
22.9.1 BY USAGE
22.9.1.1. MEDICATION AUGMENTATION
22.9.1.2. MEDICATION REPLACEMENT
22.9.2 BY TYPE
22.9.2.1. OUTPATIENT TREATMENT
22.9.2.1.1. COGNITIVE BEHAVIORAL THERAPY (CBT)
22.9.2.1.2. FLUENCY TRANING
22.9.2.1.3. CONTIGENCY MANAGEMENT
22.9.2.1.4. CRAVING AND TRIGGER ASSESSMENT
22.9.2.1.5. TRANSMUCOSAL BUPRENORPHINE
22.9.2.1.6. OTHERS
22.9.2.2. MONOTHERAPY
22.9.2.2.1. INSOMNIA-SPECIFIC COGNITIVE BEHAVIORAL THERAPY (CBTI)
22.9.2.2.2. COGNITIVE RESTRUCTURING
22.9.2.2.3. OTHERS
22.9.2.3. OTHERS
22.1 MOVEMENT DISORDER
22.10.1 BY USAGE
22.10.1.1. MEDICATION AUGMENTATION
22.10.1.2. MEDICATION REPLACEMENT
22.10.2 BY TYPE
22.10.2.1. OUTPATIENT TREATMENT
22.10.2.1.1. COGNITIVE BEHAVIORAL THERAPY (CBT)
22.10.2.1.2. FLUENCY TRANING
22.10.2.1.3. CONTIGENCY MANAGEMENT
22.10.2.1.4. CRAVING AND TRIGGER ASSESSMENT
22.10.2.1.5. TRANSMUCOSAL BUPRENORPHINE
22.10.2.1.6. OTHERS
22.10.2.2. MONOTHERAPY
22.10.2.2.1. INSOMNIA-SPECIFIC COGNITIVE BEHAVIORAL THERAPY (CBTI)
22.10.2.2.2. COGNITIVE RESTRUCTURING
22.10.2.2.3. OTHERS
22.10.2.3. OTHERS
22.11 MULTIPLE SCLEROSIS
22.11.1 BY USAGE
22.11.1.1. MEDICATION AUGMENTATION
22.11.1.2. MEDICATION REPLACEMENT
22.11.2 BY TYPE
22.11.2.1. OUTPATIENT TREATMENT
22.11.2.1.1. COGNITIVE BEHAVIORAL THERAPY (CBT)
22.11.2.1.2. FLUENCY TRANING
22.11.2.1.3. CONTIGENCY MANAGEMENT
22.11.2.1.4. CRAVING AND TRIGGER ASSESSMENT
22.11.2.1.5. TRANSMUCOSAL BUPRENORPHINE
22.11.2.1.6. OTHERS
22.11.2.2. MONOTHERAPY
22.11.2.2.1. INSOMNIA-SPECIFIC COGNITIVE BEHAVIORAL THERAPY (CBTI)
22.11.2.2.2. COGNITIVE RESTRUCTURING
22.11.2.2.3. OTHERS
22.11.2.3. OTHERS
22.12 MIGRAINE
22.12.1 BY USAGE
22.12.1.1. MEDICATION AUGMENTATION
22.12.1.2. MEDICATION REPLACEMENT
22.12.2 BY TYPE
22.12.2.1. OUTPATIENT TREATMENT
22.12.2.1.1. COGNITIVE BEHAVIORAL THERAPY (CBT)
22.12.2.1.2. FLUENCY TRANING
22.12.2.1.3. CONTIGENCY MANAGEMENT
22.12.2.1.4. CRAVING AND TRIGGER ASSESSMENT
22.12.2.1.5. TRANSMUCOSAL BUPRENORPHINE
22.12.2.1.6. OTHERS
22.12.2.2. MONOTHERAPY
22.12.2.2.1. INSOMNIA-SPECIFIC COGNITIVE BEHAVIORAL THERAPY (CBTI)
22.12.2.2.2. COGNITIVE RESTRUCTURING
22.12.2.2.3. OTHERS
22.12.2.3. OTHERS
22.13 AUSTISM SPECTRUM DISORDER
22.13.1 BY USAGE
22.13.1.1. MEDICATION AUGMENTATION
22.13.1.2. MEDICATION REPLACEMENT
22.13.2 BY TYPE
22.13.2.1. OUTPATIENT TREATMENT
22.13.2.1.1. COGNITIVE BEHAVIORAL THERAPY (CBT)
22.13.2.1.2. FLUENCY TRANING
22.13.2.1.3. CONTIGENCY MANAGEMENT
22.13.2.1.4. CRAVING AND TRIGGER ASSESSMENT
22.13.2.1.5. TRANSMUCOSAL BUPRENORPHINE
22.13.2.1.6. OTHERS
22.13.2.2. MONOTHERAPY
22.13.2.2.1. INSOMNIA-SPECIFIC COGNITIVE BEHAVIORAL THERAPY (CBTI)
22.13.2.2.2. COGNITIVE RESTRUCTURING
22.13.2.2.3. OTHERS
22.13.2.3. OTHERS
22.14 ONCLOGY
22.14.1 BY USAGE
22.14.1.1. MEDICATION AUGMENTATION
22.14.1.2. MEDICATION REPLACEMENT
22.14.2 BY TYPE
22.14.2.1. OUTPATIENT TREATMENT
22.14.2.1.1. COGNITIVE BEHAVIORAL THERAPY (CBT)
22.14.2.1.2. FLUENCY TRANING
22.14.2.1.3. CONTIGENCY MANAGEMENT
22.14.2.1.4. CRAVING AND TRIGGER ASSESSMENT
22.14.2.1.5. TRANSMUCOSAL BUPRENORPHINE
22.14.2.1.6. OTHERS
22.14.2.2. MONOTHERAPY
22.14.2.2.1. INSOMNIA-SPECIFIC COGNITIVE BEHAVIORAL THERAPY (CBTI)
22.14.2.2.2. COGNITIVE RESTRUCTURING
22.14.2.2.3. OTHERS
22.14.2.3. OTHERS
22.15 INFLAMMATION
22.15.1 BY USAGE
22.15.1.1. MEDICATION AUGMENTATION
22.15.1.2. MEDICATION REPLACEMENT
22.15.2 BY TYPE
22.15.2.1. OUTPATIENT TREATMENT
22.15.2.1.1. COGNITIVE BEHAVIORAL THERAPY (CBT)
22.15.2.1.2. FLUENCY TRANING
22.15.2.1.3. CONTIGENCY MANAGEMENT
22.15.2.1.4. CRAVING AND TRIGGER ASSESSMENT
22.15.2.1.5. TRANSMUCOSAL BUPRENORPHINE
22.15.2.1.6. OTHERS
22.15.2.2. MONOTHERAPY
22.15.2.2.1. INSOMNIA-SPECIFIC COGNITIVE BEHAVIORAL THERAPY (CBTI)
22.15.2.2.2. COGNITIVE RESTRUCTURING
22.15.2.2.3. OTHERS
22.15.2.3. OTHERS
22.16 RESPIRATORY
22.16.1 BY USAGE
22.16.1.1. MEDICATION AUGMENTATION
22.16.1.2. MEDICATION REPLACEMENT
22.16.2 BY TYPE
22.16.2.1. OUTPATIENT TREATMENT
22.16.2.1.1. COGNITIVE BEHAVIORAL THERAPY (CBT)
22.16.2.1.2. FLUENCY TRANING
22.16.2.1.3. CONTIGENCY MANAGEMENT
22.16.2.1.4. CRAVING AND TRIGGER ASSESSMENT
22.16.2.1.5. TRANSMUCOSAL BUPRENORPHINE
22.16.2.1.6. OTHERS
22.16.2.2. MONOTHERAPY
22.16.2.2.1. INSOMNIA-SPECIFIC COGNITIVE BEHAVIORAL THERAPY (CBTI)
22.16.2.2.2. COGNITIVE RESTRUCTURING
22.16.2.2.3. OTHERS
22.16.2.3. OTHERS
22.17 CARDIOVASACULAR
22.17.1 BY USAGE
22.17.1.1. MEDICATION AUGMENTATION
22.17.1.2. MEDICATION REPLACEMENT
22.17.2 BY TYPE
22.17.2.1. OUTPATIENT TREATMENT
22.17.2.1.1. COGNITIVE BEHAVIORAL THERAPY (CBT)
22.17.2.1.2. FLUENCY TRANING
22.17.2.1.3. CONTIGENCY MANAGEMENT
22.17.2.1.4. CRAVING AND TRIGGER ASSESSMENT
22.17.2.1.5. TRANSMUCOSAL BUPRENORPHINE
22.17.2.1.6. OTHERS
22.17.2.2. MONOTHERAPY
22.17.2.2.1. INSOMNIA-SPECIFIC COGNITIVE BEHAVIORAL THERAPY (CBTI)
22.17.2.2.2. COGNITIVE RESTRUCTURING
22.17.2.2.3. OTHERS
22.17.2.3. OTHERS
22.18 PAIN MANAGEMENT
22.18.1 BY USAGE
22.18.1.1. MEDICATION AUGMENTATION
22.18.1.2. MEDICATION REPLACEMENT
22.18.2 BY TYPE
22.18.2.1. OUTPATIENT TREATMENT
22.18.2.1.1. COGNITIVE BEHAVIORAL THERAPY (CBT)
22.18.2.1.2. FLUENCY TRANING
22.18.2.1.3. CONTIGENCY MANAGEMENT
22.18.2.1.4. CRAVING AND TRIGGER ASSESSMENT
22.18.2.1.5. TRANSMUCOSAL BUPRENORPHINE
22.18.2.1.6. OTHERS
22.18.2.2. MONOTHERAPY
22.18.2.2.1. INSOMNIA-SPECIFIC COGNITIVE BEHAVIORAL THERAPY (CBTI)
22.18.2.2.2. COGNITIVE RESTRUCTURING
22.18.2.2.3. OTHERS
22.18.2.3. OTHERS
22.19 METABOLIC CONDITIONS
22.19.1 BY USAGE
22.19.1.1. MEDICATION AUGMENTATION
22.19.1.2. MEDICATION REPLACEMENT
22.19.2 BY TYPE
22.19.2.1. OUTPATIENT TREATMENT
22.19.2.1.1. COGNITIVE BEHAVIORAL THERAPY (CBT)
22.19.2.1.2. FLUENCY TRANING
22.19.2.1.3. CONTIGENCY MANAGEMENT
22.19.2.1.4. CRAVING AND TRIGGER ASSESSMENT
22.19.2.1.5. TRANSMUCOSAL BUPRENORPHINE
22.19.2.1.6. OTHERS
22.19.2.2. MONOTHERAPY
22.19.2.2.1. INSOMNIA-SPECIFIC COGNITIVE BEHAVIORAL THERAPY (CBTI)
22.19.2.2.2. COGNITIVE RESTRUCTURING
22.19.2.2.3. OTHERS
22.19.2.3. OTHERS
22.2 OTHERS
23 GLOBAL PRESCRIPTION DIGITAL THERAPEUTICS (DTX) MARKET, BY PATIENTS
23.1 OVERVIEW
23.2 CHILDREN
23.3 ADULTS
24 GLOBAL CELL ANALYSIS MARKET, BY GEOGRAPHY
GLOBAL CELL ANALYSIS MARKET (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
24.1 NORTH AMERICA
24.1.1 U.S.
24.1.2 CANADA
24.1.3 MEXICO
24.2 EUROPE
24.2.1 GERMANY
24.2.2 FRANCE
24.2.3 U.K.
24.2.4 ITALY
24.2.5 SPAIN
24.2.6 RUSSIA
24.2.7 TURKEY
24.2.8 BELGIUM
24.2.9 NETHERLANDS
24.2.10 SWITZERLAND
24.2.11 REST OF EUROPE
24.3 ASIA-PACIFIC
24.3.1 JAPAN
24.3.2 CHINA
24.3.3 SOUTH KOREA
24.3.4 INDIA
24.3.5 AUSTRALIA
24.3.6 SINGAPORE
24.3.7 THAILAND
24.3.8 MALAYSIA
24.3.9 INDONESIA
24.3.10 PHILIPPINES
24.3.11 REST OF ASIA-PACIFIC
24.4 SOUTH AMERICA
24.4.1 BRAZIL
24.4.2 ARGENTINA
24.4.3 PERU
24.4.4 CHILE
24.4.5 COLOMBIA
24.4.6 VENEZUELA
24.4.7 REST OF SOUTH AMERICA
24.5 MIDDLE EAST AND AFRICA
24.5.1 SOUTH AFRICA
24.5.2 SAUDI ARABIA
24.5.3 UAE
24.5.4 EGYPT
24.5.5 ISRAEL
24.5.6 REST OF MIDDLE EAST AND AFRICA
24.6 KEY PRIMARY INSIGHTS: BY MAJOR COUNTRIES
25 GLOBAL CELL ANALYSIS MARKET, COMPANY LANDSCAPE
25.1 COMPANY SHARE ANALYSIS: GLOBAL
25.2 COMPANY SHARE ANALYSIS: NORTH AMERICA
25.3 COMPANY SHARE ANALYSIS: EUROPE
25.4 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
25.5 MERGERS & ACQUISITIONS
25.6 NEW PRODUCT DEVELOPMENT & APPROVALS
25.7 EXPANSIONS
25.8 REGULATORY CHANGES
25.9 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
26 GLOBAL CELL ANALYSIS MARKET, SWOT AND DBMR ANALYSIS
27 GLOBAL CELL ANALYSIS MARKET, COMPANY PROFILE
27.1 PEAR THERAPEUTICS, INC.
27.1.1 COMPANY OVERVIEW
27.1.2 REVENUE ANALYSIS
27.1.3 GEOGRAPHIC PRESENCE
27.1.4 PRODUCT PORTFOLIO
27.1.5 RECENT DEVELOPEMENTS
27.2 AKILI INTERACTIVE LABS, INC.
27.2.1 COMPANY OVERVIEW
27.2.2 REVENUE ANALYSIS
27.2.3 GEOGRAPHIC PRESENCE
27.2.4 PRODUCT PORTFOLIO
27.2.5 RECENT DEVELOPEMENTS
27.3 CLICK THERAPEUTICS, INC.
27.3.1 COMPANY OVERVIEW
27.3.2 REVENUE ANALYSIS
27.3.3 GEOGRAPHIC PRESENCE
27.3.4 PRODUCT PORTFOLIO
27.3.5 RECENT DEVELOPEMENTS
27.4 BETTER THERAPEUTICS, LLC
27.4.1 COMPANY OVERVIEW
27.4.2 REVENUE ANALYSIS
27.4.3 GEOGRAPHIC PRESENCE
27.4.4 PRODUCT PORTFOLIO
27.4.5 RECENT DEVELOPEMENTS
27.5 XEALTH
27.5.1 COMPANY OVERVIEW
27.5.2 REVENUE ANALYSIS
27.5.3 GEOGRAPHIC PRESENCE
27.5.4 PRODUCT PORTFOLIO
27.5.5 RECENT DEVELOPEMENTS
27.6 KAIA HEALTH
27.6.1 COMPANY OVERVIEW
27.6.2 REVENUE ANALYSIS
27.6.3 GEOGRAPHIC PRESENCE
27.6.4 PRODUCT PORTFOLIO
27.6.5 RECENT DEVELOPEMENTS
27.7 OMADA HEALTH, INC.
27.7.1 COMPANY OVERVIEW
27.7.2 REVENUE ANALYSIS
27.7.3 GEOGRAPHIC PRESENCE
27.7.4 PRODUCT PORTFOLIO
27.7.5 RECENT DEVELOPEMENTS
27.8 WELLDOC
27.8.1 COMPANY OVERVIEW
27.8.2 REVENUE ANALYSIS
27.8.3 GEOGRAPHIC PRESENCE
27.8.4 PRODUCT PORTFOLIO
27.8.5 RECENT DEVELOPEMENTS
27.9 VOLUNTIS
27.9.1 COMPANY OVERVIEW
27.9.2 REVENUE ANALYSIS
27.9.3 GEOGRAPHIC PRESENCE
27.9.4 PRODUCT PORTFOLIO
27.9.5 RECENT DEVELOPEMENTS
27.1 SOLERA NETWORK
27.10.1 COMPANY OVERVIEW
27.10.2 REVENUE ANALYSIS
27.10.3 GEOGRAPHIC PRESENCE
27.10.4 PRODUCT PORTFOLIO
27.10.5 RECENT DEVELOPEMENTS
27.11 2MORROW INC.
27.11.1 COMPANY OVERVIEW
27.11.2 REVENUE ANALYSIS
27.11.3 GEOGRAPHIC PRESENCE
27.11.4 PRODUCT PORTFOLIO
27.11.5 RECENT DEVELOPEMENTS
27.12 GAIA
27.12.1 COMPANY OVERVIEW
27.12.2 REVENUE ANALYSIS
27.12.3 GEOGRAPHIC PRESENCE
27.12.4 PRODUCT PORTFOLIO
27.12.5 RECENT DEVELOPEMENTS
27.13 RESMED
27.13.1 COMPANY OVERVIEW
27.13.2 REVENUE ANALYSIS
27.13.3 GEOGRAPHIC PRESENCE
27.13.4 PRODUCT PORTFOLIO
27.13.5 RECENT DEVELOPEMENTS
27.14 DTHERA SCIENCES
27.14.1 COMPANY OVERVIEW
27.14.2 REVENUE ANALYSIS
27.14.3 GEOGRAPHIC PRESENCE
27.14.4 PRODUCT PORTFOLIO
27.14.5 RECENT DEVELOPEMENTS
27.15 SAMSUNG
27.15.1 COMPANY OVERVIEW
27.15.2 REVENUE ANALYSIS
27.15.3 GEOGRAPHIC PRESENCE
27.15.4 PRODUCT PORTFOLIO
27.15.5 RECENT DEVELOPEMENTS
27.16 BIGHEALTH
27.16.1 COMPANY OVERVIEW
27.16.2 REVENUE ANALYSIS
27.16.3 GEOGRAPHIC PRESENCE
27.16.4 PRODUCT PORTFOLIO
27.16.5 RECENT DEVELOPEMENTS
27.17 PROPELLER HEALTH
27.17.1 COMPANY OVERVIEW
27.17.2 REVENUE ANALYSIS
27.17.3 GEOGRAPHIC PRESENCE
27.17.4 PRODUCT PORTFOLIO
27.17.5 RECENT DEVELOPEMENTS
27.18 F. HOFFMANN-LA ROCHE LTD
27.18.1 COMPANY OVERVIEW
27.18.2 REVENUE ANALYSIS
27.18.3 GEOGRAPHIC PRESENCE
27.18.4 PRODUCT PORTFOLIO
27.18.5 RECENT DEVELOPEMENTS
27.19 LIMBIX HEALTH, INC
27.19.1 COMPANY OVERVIEW
27.19.2 REVENUE ANALYSIS
27.19.3 GEOGRAPHIC PRESENCE
27.19.4 PRODUCT PORTFOLIO
27.19.5 RECENT DEVELOPEMENTS
27.2 NUVOAIR AB
27.20.1 COMPANY OVERVIEW
27.20.2 REVENUE ANALYSIS
27.20.3 GEOGRAPHIC PRESENCE
27.20.4 PRODUCT PORTFOLIO
27.20.5 RECENT DEVELOPEMENTS
27.21 ALTRAN
27.21.1 COMPANY OVERVIEW
27.21.2 REVENUE ANALYSIS
27.21.3 GEOGRAPHIC PRESENCE
27.21.4 PRODUCT PORTFOLIO
27.21.5 RECENT DEVELOPEMENTS
27.22 SENSYNE HEALTH PLC
27.22.1 COMPANY OVERVIEW
27.22.2 REVENUE ANALYSIS
27.22.3 GEOGRAPHIC PRESENCE
27.22.4 PRODUCT PORTFOLIO
27.22.5 RECENT DEVELOPEMENTS
27.23 BIOFOURMIS
27.23.1 COMPANY OVERVIEW
27.23.2 REVENUE ANALYSIS
27.23.3 GEOGRAPHIC PRESENCE
27.23.4 PRODUCT PORTFOLIO
27.23.5 RECENT DEVELOPEMENTS
27.24 SMARTPATIENT GMBH
27.24.1 COMPANY OVERVIEW
27.24.2 REVENUE ANALYSIS
27.24.3 GEOGRAPHIC PRESENCE
27.24.4 PRODUCT PORTFOLIO
27.24.5 RECENT DEVELOPEMENTS
28 CONCLUSION
29 QUESTIONNAIRE
30 ABOUT DATA BRIDGE MARKET RESEARCH

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.