Im Jahr 2020 zeigten die Daten von UNAIDS zur globalen HIV- und AIDS-Statistik, dass 37,7 Millionen [30,2–45,1 Millionen] Menschen mit HIV lebten. Davon waren rund 36 Millionen Erwachsene betroffen. Rund 1,7 Millionen Kinder im Alter von 14 Jahren waren mit HIV infiziert, und bei 17,8 Millionen Frauen wurde HIV diagnostiziert.
Vollständigen Bericht abrufen unter https://www.databridgemarketresearch.com/reports/global-rapid-diagnostic-tests-rdt-market
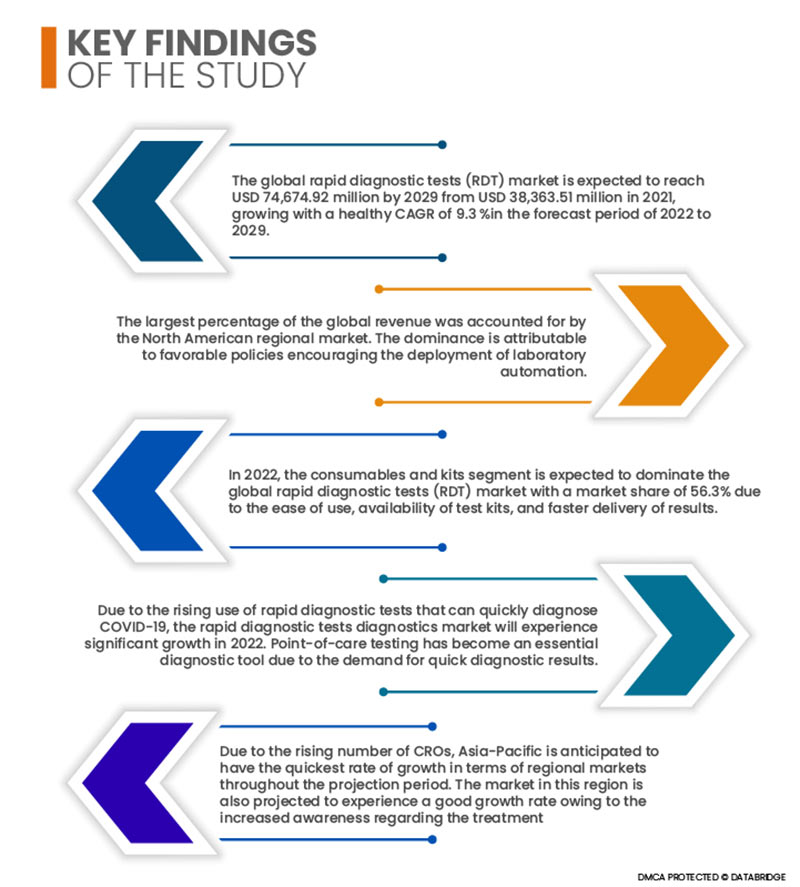
Der globale Markt für Schnelldiagnostiktests (RDT) soll von 38.363,51 Millionen USD im Jahr 2021 auf 74.674,92 Millionen USD im Jahr 2029 anwachsen und im Prognosezeitraum 2022 bis 2029 eine gesunde durchschnittliche jährliche Wachstumsrate (CAGR) von 9,3 % aufweisen. Die Expansion dieses Marktes ist auf mehrere Faktoren zurückzuführen, darunter der wachsende Bedarf an der Einführung von LIMS, die zunehmende Nutzung von Schnelldiagnostik zur Einhaltung strenger gesetzlicher Anforderungen, steigende Ausgaben für Forschung und Entwicklung in verschiedenen Branchen und zunehmende staatliche Unterstützung.
Die weltweite Zunahme chronischer Krankheiten wird das Marktwachstum antreiben
Schnelltests sind für die Diagnose chronischer Infektionskrankheiten wie dem Humanen Immundefizienz-Virus (HIV) und Malaria unerlässlich. Beide Erkrankungen weisen unspezifische Symptome auf. Schnelltests reduzieren die Anzahl der Besuche in Diagnosezentren zur Ergebniserfassung. Dadurch verbessern sie die Diagnosespezifität, verringern die mutmaßliche Behandlungsabhängigkeit und verringern das Risiko, vor der korrekten Diagnose krank zu werden. Dies schafft Bewusstsein und dürfte die Nachfrage nach Forschung und Entwicklung von Schnelltestkits unter Wissenschaftlern erhöhen. Dies würde eine rechtzeitige Behandlung von Patienten mit chronischen Erkrankungen ermöglichen.
Berichtsumfang und Marktsegmentierung
Berichtsmetrik
|
Details
|
Prognosezeitraum
|
2022 bis 2029
|
Basisjahr
|
2021
|
Historische Jahre
|
2020 (Anpassbar auf 2014 – 2019)
|
Quantitative Einheiten
|
Umsatz in Millionen USD, Mengen in Einheiten, Preise in USD
|
Abgedeckte Segmente
|
Produkttyp (Verbrauchsmaterialien und Kits, Instrumente und Sonstiges), Modus (Professionelles Schnelldiagnostikprodukt und rezeptfreies Schnelldiagnostikprodukt [OTC]), Technologie (PCR-basiert, Durchflussassays, Lateral-Flow-Immunchromatographie-Assays, Agglutinationsassay, Mikrofluidik, Substrattechnologie und Sonstiges), Modalität (Laborbasierter Test und Nicht-Laborbasierter Test), Altersgruppe (Erwachsene und Kinder), Testtyp (Bestätigungsbestimmung, serologische Tests und Virussequenzierung), Ansatz (In-vitro-Diagnostik, Molekulardiagnostik), Probe (Abstrich, Blut, Urin, Speichel, Sputum und Sonstiges), Anwendung (Tests auf Infektionskrankheiten, Glukoseüberwachung, kardiologische Tests, onkologische Tests, kardiometabolische Tests, Drogentests, Schwangerschafts- und Fruchtbarkeitstests, toxikologische Tests, Sonstiges), Endbenutzer (Krankenhaus und Klinik, Diagnoselabor, häusliche Pflege, Forschung und Lehre Institute und andere), Vertriebskanal (Direktausschreibung, Einzelhandelsverkauf, andere)
|
Abgedeckte Länder
|
USA, Kanada und Mexiko in Nordamerika, Deutschland, Frankreich, Großbritannien, Niederlande, Schweiz, Belgien, Russland, Italien, Spanien, Türkei, Restliches Europa in Europa, China, Japan, Indien, Südkorea, Singapur, Malaysia, Australien, Thailand, Indonesien, Philippinen, Restlicher Asien-Pazifik-Raum (APAC) im Asien-Pazifik-Raum (APAC), Saudi-Arabien, Vereinigte Arabische Emirate, Südafrika, Ägypten, Israel, Restlicher Naher Osten und Afrika (MEA) als Teil des Nahen Ostens und Afrikas (MEA), Brasilien, Argentinien und Restliches Südamerika als Teil von Südamerika.
|
Abgedeckte Marktteilnehmer
|
Abbott (USA), Danaher (USA), Cellex (Italien), AdvaCare Pharma (Indien), Access Bio (USA), Cardinal Health (USA), Bio-Rad Laboratories, Inc. (USA), BD (USA), F. Hoffmann-La Roche Ltd (Schweiz), bioMérieux SA (Frankreich), InBios International, Inc. (USA), Gnomegen LLC (USA), QIAGEN (Deutschland), Quidel Corporation (USA), Chembio Diagnostics Systems, Inc. (USA), Siemens Healthcare GmbH (Deutschland), PerkinElmer Inc. (USA), Sekisui Diagnostics (USA), Fujirebio (Japan), PTS Diagnostics (USA), LamdaGen Corporation (USA), Werfen (Spanien), Nova Biomedical (USA), Trinity Biotech (Irland), Sysmex Europe GmbH (Deutschland), Luminex Corporation (USA), MEGAKOR DIAGNOSTIK GMBH (Österreich) und andere.
|
Im Bericht behandelte Datenpunkte
|
Zusätzlich zu den Markteinblicken wie Marktwert, Wachstumsrate, Marktsegmenten, geografischer Abdeckung, Marktteilnehmern und Marktszenario enthält der vom Marktforschungsteam von Data Bridge kuratierte Marktbericht eine eingehende Expertenanalyse, Patientenepidemiologie, Pipeline-Analyse, Preisanalyse und regulatorische Rahmenbedingungen.
|
Segmentanalyse:
Der globale Markt für Schnelldiagnosetests (RDT) ist basierend auf Produkttyp, Modus, Technologie, Modalität, Altersgruppe, Testtyp, Ansatz, Probe, Anwendung, Endbenutzer und Vertriebskanal in elf wichtige Segmente unterteilt.
- Der globale Markt für Schnelldiagnostiktests (RDT) ist nach Produkttyp in Verbrauchsmaterialien und Kits, Instrumente und Sonstiges unterteilt. Im Jahr 2022 wird das Segment Verbrauchsmaterialien und Kits voraussichtlich den globalen Markt für Schnelldiagnostiktests (RDT) mit einem Marktanteil von 56,3 % dominieren. Dies ist auf die einfache Handhabung, die Verfügbarkeit von Testkits und die schnellere Ergebnisbereitstellung zurückzuführen.
- Der globale Markt für Schnelltests (RDT) ist nach Anwendungsgebiet segmentiert: professionelle Schnelltests und rezeptfreie Schnelltests. Im Jahr 2022 wird das Segment der professionellen Schnelltests voraussichtlich den globalen Markt für Schnelltests (RDT) mit einem Marktanteil von 76,4 % dominieren. Dies ist auf die Genauigkeit und die zunehmende Nutzung in Behandlungszentren, beispielsweise zu Hause, zurückzuführen.
- Der globale Markt für Schnelldiagnostiktests (RDT) ist technologisch in PCR-basierte, Durchfluss-, Lateral-Flow-Immunochromatographie-, Agglutinations-, Mikrofluidik- und Substrattechnologie-Verfahren unterteilt. Im Jahr 2022 wird das PCR-basierte Segment voraussichtlich den globalen Markt für Schnelldiagnostiktests (RDT) mit einem Marktanteil von 39,31 % dominieren. Dies ist auf Geschwindigkeit, Zuverlässigkeit, schnellere Ergebnislieferung und quantitative Kapazität zurückzuführen.
- Der globale Markt für Schnelldiagnostiktests (RDT) wird nach Modalität in laborbasierte und nicht-laborbasierte Tests unterteilt. Im Jahr 2022 wird das Segment der laborbasierten Tests voraussichtlich den globalen Markt für Schnelldiagnostiktests (RDT) mit einem Marktanteil von 71,6 % dominieren. Dies ist auf die Sicherheit, die Verfügbarkeit von Laboren, die Einhaltung der gesetzlichen Richtlinien und die Kosteneinsparungen zurückzuführen.
- Der globale Markt für Schnelldiagnosetests (RDT) ist nach Altersgruppen in Erwachsene und Kinder unterteilt. Aufgrund der Zunahme geriatrischer Bevölkerungsgruppen und chronischer Infektionskrankheiten wird erwartet, dass das Erwachsenensegment im Jahr 2022 den globalen Markt für Schnelldiagnosetests (RDT) mit einem Marktanteil von 85,1 % dominieren wird.
- Der globale Markt für Schnelldiagnostiktests (RDT) ist nach Testtyp in die Bereiche Bestätigung, serologische Tests und Virussequenzierung unterteilt. Im Jahr 2022 wird das Segment Bestätigung voraussichtlich mit einem Marktanteil von 43,6 % den globalen Markt für Schnelldiagnostiktests (RDT) dominieren, da die Diagnose mittels RDTs zur Bestimmung der Ursache von Infektionskrankheiten erforderlich ist, da die Diagnose mittels RDTs bestätigte Ergebnisse liefert und die RDTs sensitiver sind.
- Der globale Markt für Schnelldiagnostiktests (RDT) wird ansatzweise in In-vitro-Diagnostik und Molekulardiagnostik unterteilt. Im Jahr 2022 wird das Segment der In-vitro-Diagnostik voraussichtlich den globalen Markt für Schnelldiagnostiktests (RDT) mit einem Marktanteil von 74,2 % dominieren. Dies ist auf die Fähigkeit der In-vitro-Diagnostik zurückzuführen, Infektionskrankheiten wie COVID-19 zu erkennen, Schwangerschafts- und Fruchtbarkeitsstörungen zu erkennen und in der Präzisionsmedizin anwendbar zu sein.
- Der globale Markt für Schnelltests (RDT) ist auf Probenbasis in Abstrich, Blut, Urin, Speichel, Sputum und weitere Verfahren unterteilt. Im Jahr 2022 wird das Abstrichsegment voraussichtlich den globalen Markt für Schnelltests (RDT) mit einem Marktanteil von 45,5 % dominieren. Dies ist auf die zunehmende Präferenz der Patienten und Fortschritte in der Serologie für die Entnahme von Abstrichen zurückzuführen.
- Der globale Markt für Schnelldiagnostiktests (RDT) ist nach Anwendung segmentiert in Infektionskrankheitstests, Glukoseüberwachung, kardiologische Tests, onkologische Tests, kardiometabolische Tests, Drogentests, Schwangerschafts- und Fruchtbarkeitstests, toxikologische Tests und weitere. Im Jahr 2022 wird das Segment der Infektionskrankheitstests voraussichtlich den globalen Markt für Schnelldiagnostiktests (RDT) mit einem Marktanteil von 34,5 % dominieren. Grund hierfür sind die steigende Zahl chronischer Infektionskrankheiten, der Anstieg der geriatrischen Bevölkerung und die staatliche Förderung von Schnelldiagnostik.
- Der globale Markt für Schnelldiagnostiktests (RDT) ist nach Endverbraucher segmentiert in Krankenhäuser und Kliniken, Diagnoselabore, häusliche Pflegeeinrichtungen, Forschungs- und Hochschuleinrichtungen und weitere. Im Jahr 2022 wird das Krankenhaus- und Kliniksegment voraussichtlich den globalen Markt für Schnelldiagnostiktests (RDT) mit einem Marktanteil von 55,1 % dominieren. Dies ist auf die Verfügbarkeit fortschrittlicher Schnelldiagnostik-Kits in Krankenhäusern und das steigende verfügbare Einkommen zurückzuführen.
Das Krankenhaus- und Kliniksegment wird das Endverbrauchersegment des Marktes für Schnelldiagnosetests (RDT) dominieren
Das Krankenhaus- und Kliniksegment wird sich als dominierendes Segment im Endverbrauchersegment herauskristallisieren. Dies liegt an der wachsenden Zahl von Krankenhäusern und Kliniken auf dem Markt, insbesondere in Entwicklungsländern. Darüber hinaus werden das Wachstum und die Ausweitung der Forschungs- und Entwicklungsdienstleistungen auf globaler Ebene das Wachstum dieses Segments weiter fördern.
- Der globale Markt für Schnelldiagnostiktests (RDT) ist nach Vertriebskanälen in Direktausschreibungen, Einzelhandelsverkäufe und andere Segmente unterteilt. Im Jahr 2022 wird das Direktausschreibungssegment voraussichtlich den globalen Markt für Schnelldiagnostiktests (RDT) mit einem Marktanteil von 61,7 % dominieren. Dies ist auf die zunehmende Anzahl vertraglicher Vereinbarungen zwischen Herstellern und Händlern für Schnelldiagnostiktests und die Beteiligung an Ausschreibungen zurückzuführen.
Das Segment der Direktausschreibungen wird das Vertriebskanalsegment des Marktes für Schnelldiagnosetests (RDT) dominieren
Das Segment der Direktausschreibungen wird sich mit einem Marktanteil von rund 62 % als dominierendes Segment im Vertriebskanal herauskristallisieren. Dies ist auf die zunehmende Zahl von Infrastrukturentwicklungsaktivitäten und stationären Einzelhändlern zurückzuführen, insbesondere in Entwicklungsländern. Darüber hinaus werden das Wachstum und die Expansion der Chemie- und Industriebranche weltweit das Wachstum dieses Segments weiter fördern.
Hauptakteure
Data Bridge Market Research erkennt die folgenden Unternehmen als Marktteilnehmer im Markt für Schnelldiagnosetests (RDT) an: Abbott (USA), Danaher (USA), Cellex (Italien), AdvaCare Pharma (Indien), Access Bio (USA), Cardinal Health (USA), Bio-Rad Laboratories, Inc. (USA), BD (USA), F. Hoffmann-La Roche Ltd (Schweiz), bioMérieux SA (Frankreich), InBios International, Inc. (USA), Gnomegen LLC (USA), QIAGEN (Deutschland), Quidel Corporation (USA), Chembio Diagnostics Systems, Inc. (USA), Siemens Healthcare GmbH (Deutschland), PerkinElmer Inc. (USA), Sekisui Diagnostics (USA), Fujirebio (Japan) und PTS Diagnostics (USA)
Marktentwicklung
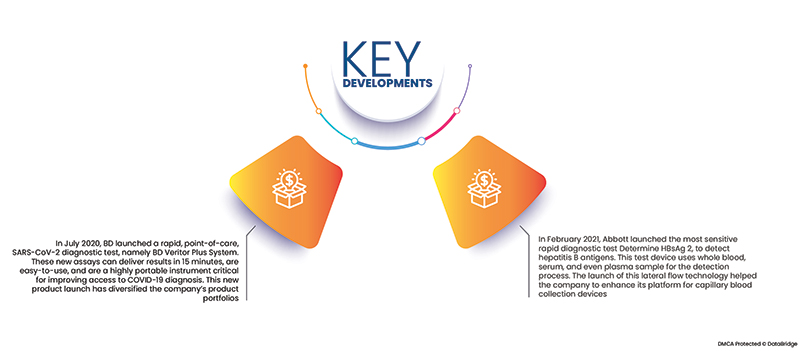
- Im Juli 2020 brachte BD mit dem BD Veritor Plus System einen schnellen Point-of-Care-Diagnosetest für SARS-CoV-2 auf den Markt. Diese neuen Tests liefern Ergebnisse in 15 Minuten, sind einfach anzuwenden und ein äußerst tragbares Instrument, das für einen verbesserten Zugang zur COVID-19-Diagnose entscheidend ist. Diese neue Produkteinführung hat das Produktportfolio des Unternehmens diversifiziert.
- Im Februar 2021 brachte Abbott den empfindlichsten Schnelltest „Determinate HBsAg 2“ zum Nachweis von Hepatitis-B-Antigenen auf den Markt. Dieses Testgerät verwendet Vollblut-, Serum- und sogar Plasmaproben zur Erkennung. Mit der Einführung dieser Lateral-Flow-Technologie konnte das Unternehmen seine Plattform für Kapillarblutentnahmegeräte erweitern.
Regionale Analyse
Geografisch betrachtet sind die im Marktbericht für Schnelldiagnosetests (RDT) abgedeckten Länder die USA, Kanada und Mexiko in Nordamerika, Deutschland, Frankreich, Großbritannien, die Niederlande, die Schweiz, Belgien, Russland, Italien, Spanien, die Türkei, das übrige Europa in Europa, China, Japan, Indien, Südkorea, Singapur, Malaysia, Australien, Thailand, Indonesien, die Philippinen, der übrige asiatisch-pazifische Raum (APAC) in der Region Asien-Pazifik (APAC), Saudi-Arabien, die Vereinigten Arabischen Emirate, Südafrika, Ägypten, Israel, der übrige Nahe Osten und Afrika (MEA) als Teil des Nahen Ostens und Afrikas (MEA), Brasilien, Argentinien und der übrige Südamerika als Teil von Südamerika.
Laut Marktforschungsanalyse von Data Bridge:
Nordamerika ist im Prognosezeitraum 2022 – 2029 die dominierende Region auf dem Markt für Schnelldiagnosetests (RDT).
Der nordamerikanische Regionalmarkt erwirtschaftete den größten Anteil am weltweiten Umsatz. Diese Dominanz ist auf eine günstige Politik zurückzuführen, die den Einsatz von Laborautomatisierung fördert. Modernste Infrastruktur, verstärkte staatliche Förderung, die Beteiligung von Unternehmen an der Entwicklung neuartiger COVID-19-Schnelltests und die steigende Nachfrage nach genetischer Forschung tragen zu diesem hohen Anteil der Region bei.
Der asiatisch-pazifische Raum wird im Prognosezeitraum 2022 – 2029 voraussichtlich die am schnellsten wachsende Region im Markt für Schnelldiagnosetests (RDT) sein .
Aufgrund der steigenden Anzahl von Auftragsforschungsinstituten wird erwartet, dass der asiatisch-pazifische Raum im Prognosezeitraum das höchste regionale Marktwachstum verzeichnet. Auch für diesen Markt wird aufgrund des gestiegenen Bewusstseins für die Behandlung von Infektionskrankheiten und günstiger Erstattungsszenarien ein gutes Wachstum prognostiziert.
Auswirkungen von COVID-19
Aufgrund der zunehmenden Nutzung von Schnelltests zur schnellen Diagnose von COVID-19 wird der Markt für Schnelltests im Jahr 2022 deutlich wachsen. Point-of-Care-Tests haben sich aufgrund der Nachfrage nach schnellen Diagnoseergebnissen zu einem unverzichtbaren Diagnoseinstrument entwickelt. Die Nachfrage nach schnellen Antigen-Testkits, die erfolgreich in Point-of-Care-Umgebungen eingesetzt werden können, ist aufgrund der rasant steigenden COVID-19-Fallzahlen und des zunehmenden Drucks auf Regierungen, das Patientenmanagement zu verbessern, gestiegen. Weitere wichtige Faktoren, die voraussichtlich zum langfristigen Wachstum des Point-of-Care-Diagnostikmarktes beitragen werden, sind die weltweit steigende Prävalenz anderer Atemwegserkrankungen, der Trend hin zu dezentraler Diagnostik und der verbesserte Zugang zu Point-of-Care-Geräten über Online-Plattformen.
Für detailliertere Informationen zum Marktbericht über Schnelldiagnosetests (RDT) klicken Sie hier – https://www.databridgemarketresearch.com/reports/global-rapid-diagnostic-tests-rdt-market










