Cancer is a leading cause of morbidity and mortality worldwide, and the incidence of cancer is rising in these regions due to factors such as population growth, aging, and lifestyle changes. Additionally, there is a growing burden of other target diseases, such as haematological disorders, which can also be treated with CAR-T cell therapy. This increasing disease burden underscores the need for innovative and effective treatments like CAR-T cell therapy, driving market growth.
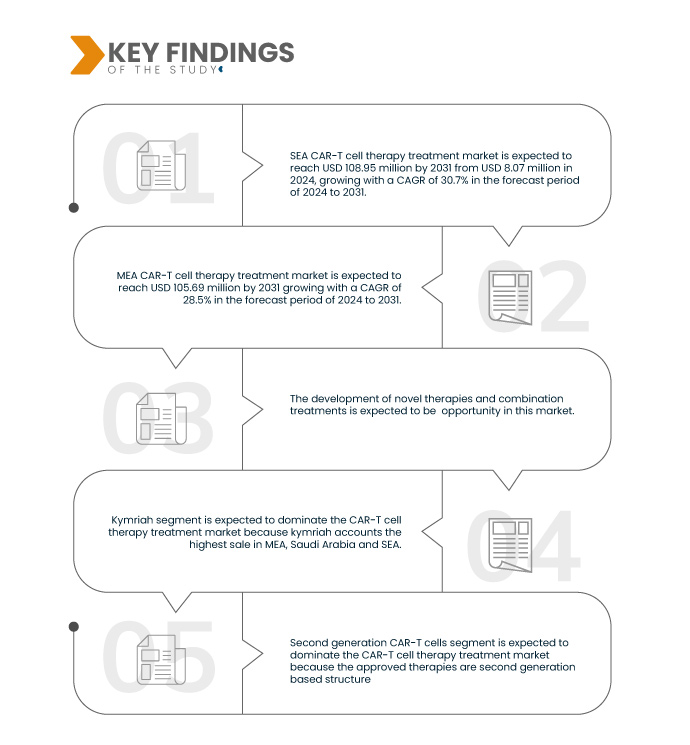
Data Bridge Market Research analyses that the SEA CAR-T Cell Therapy Treatment Market is expected to reach USD 108.95 million by 2031, growing with a CAGR of 30.7% in the forecast period of 2024 to 2031.
Access Full Report @ https://www.databridgemarketresearch.com/reports/middle-east-and-africa-car-t-cell-therapy-treatment-market
Data Bridge Market Research analyses that the MEA CAR-T Cell Therapy Treatment Market is expected to reach USD 105.69 million by 2031 growing with a CAGR of 28.5% in the forecast period of 2027 to 2031.
Key Findings of the Study
- Increase Awareness and Acceptance of Car-T Cell Therapy
The growing awareness and acceptance of CAR-T cell therapy as an effective treatment in the MEA, Saudi Arabia, and SEA regions are driven by various factors. These include heightened media coverage, educational efforts by healthcare organizations, and the sharing of success stories that highlight the therapy's effectiveness. Consequently, patients and healthcare providers are gaining a better understanding of CAR-T cell therapy's potential advantages, such as higher response rates and long-lasting remissions, especially in cases that are refractory or have relapsed. Additionally, regulatory approvals and endorsements in these regions have cemented CAR-T cell therapy's status as a legitimate and viable treatment option.
Report Scope and Market Segmentation
|
Report Metric
|
Details
|
|
Forecast Period
|
2024 to 2031
|
|
Base Year
|
2023
|
|
Historic Years
|
2022 (Customizable to 2016-2021)
|
|
Quantitative Units
|
Revenue in USD Million
|
|
Segments Covered
|
Product (Autologous CAR-T Cells and Allogeneic CAR-T Cells), Structure (First Generation CAR-T Cells, Second Generation Car-T Cells, Third Generation CAR-T Cells, and Fourth Generation CAR-T Cells), Targeted Antigens (Antigens On Solid Tumors, Antigens On Hematologic Malignancies, and Others), Brand (Yescarta, Kymriah, Tecartus, and Others), Therapeutic Application (Diffuse Large B-Cell Lymphoma, Follicular Lymphoma, Acute Lymphoblastic Leukaemia (ALL), Mantle Cell Lymphoma, Multiple Myeloma, Hematologic Malignancies, Lung Cancer, Chronic Lymphocytic Leukaemia, Gastric Cancer, Pancreatic Cancer, Breast Cancer, and Others), End User (Hospitals, Specialty Clinics, and Others), Distribution Channel (Hospitals Pharmacy and Others)
|
|
Countries Covered
|
Singapore, Indonesia, Thailand, Malaysia, Philippines, Vietnam, Saudi Arabia, South Africa, U.A.E., Israel, Kuwait, Egypt, and Rest of Middle East and Africa
|
|
Market Players Covered
|
Novartis AG (Switzerland), Gilead Sciences, Inc. (U.S.), Bristol-Myers Squibb Company (U.S.), and Johnson & Johnson Services, Inc. (U.S.), among others
|
|
Data Points Covered in the Report
|
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework
|
Segment Analysis
The MEA, Saudi Arabia and SEA CAR-T cell therapy treatment market is categorized into categorized into seven notable segments which are based on the product, structure, targeted antigens, brand, therapeutic application, end user and distribution channel.
- On the basis of product, market is segmented into autologous CAR-T cells and allogeneic CAR-T cells
Autologous CAR-T cells segment is expected to dominate the CAR-T cell therapy treatment MEA, Saudi Arabia and SEA CAR-T Cell Therapy Treatment Market
Autologous CAR-T cells segment is expected to dominate the market due to the drugs approved for CAR-T cell therapy are autologous in nature. These cells are easy to manufacture because they are derived from the patient own cells or plasma. Moreover, intensive research is ongoing on autologous cells.
- On the basis of structure, market is segmented into first generation CAR-T cells, second generation CAR-T cells, third generation CAR-T cells and fourth generation CAR-T cells
Second generation CAR-T cells segment is expected to dominate the MEA, Saudi Arabia and SEA CAR-T Cell Therapy Treatment Market
Second generation CAR-T cells segment is expected to dominate the market due to accuracy and increased use in points of care, such as homes the approved therapies are second generation based structure. Moreover, second generation are highly specific in nature which recognizes and binds tumor specific antigens.
- On the basis of targeted antigens, market is segmented into antigens on solid tumors, antigens on hematologic malignancies and others. Antigens on hematologic malignancies segment is expected to dominate market due to drugs approved for CAR-T cell therapy are majorly used for hematologic malignancies and ongoing extensive research development
- On the basis of brand, market is segmented into yescarta, kymriah, tecartus, and others. Kymriah segment is expected to dominate the market due kymriah accounts the highest sale in MEA, Saudi Arabia and SEA
- On the basis of therapeutic application, market is segmented into diffuse large B-cell lymphoma, follicular lymphoma, Acute Lymphoblastic Leukaemia (ALL), mantle cell lymphoma, multiple myeloma, hematologic malignancies, lung cancer, chronic lymphocytic leukemia, gastric cancer, pancreatic cancer, breast cancer, and others. Diffuse large B-cell lymphoma segment is expected to dominate the CAR-T cell therapy treatment market due to accounts the major revenue in the market and the follicular lymphoma is the major indication for yescarta
- On the basis of end user, market is segmented into hospitals, specialty clinics, and others. Hospitals segment is dominating the CAR-T cell therapy treatment due CAR-T cell therapy required highly trained professionals with advanced technology laboratories. So, the hospitals have all the advanced equipment and technology with trained professionals
- On the basis of distribution channel, market is segmented into hospitals pharmacy and others. Hospitals pharmacy segment is expected to dominate the CAR-T cell therapy treatment market due hospitals pharmacy are the central part of the hospitals, all the infusion related and medication related delivery sold by the pharmacy department
Major Players
Data Bridge Market Research analyzes Novartis AG (Switzerland), Gilead Sciences, Inc. (U.S.), Bristol-Myers Squibb Company (U.S.), and Johnson & Johnson Services, Inc. (U.S.) as major market players in this market.
Market Developments
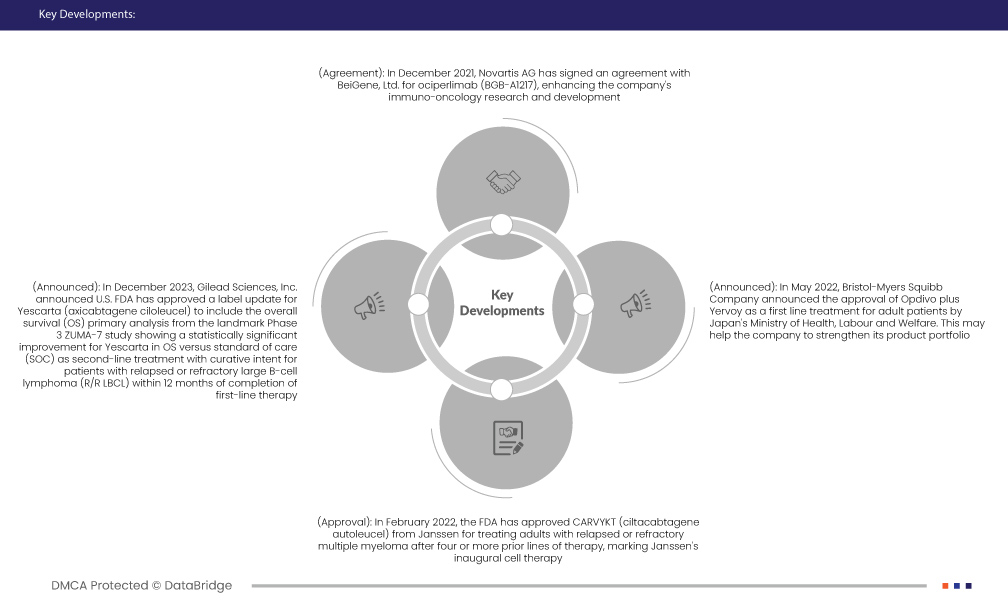
- In December 2021, Novartis AG has signed an agreement with BeiGene, Ltd. for ociperlimab (BGB-A1217), enhancing the company's immuno-oncology research and development. This collaboration contributes to Novartis Oncology's broader initiative to advance innovation in cancer treatments by incorporating a potentially transformative therapy into its expanding immunotherapy platform
- In December 2023, Gilead Sciences, Inc. announced U.S. FDA has approved a label update for Yescarta (axicabtagene ciloleucel) to include the overall survival (OS) primary analysis from the landmark Phase 3 ZUMA-7 study showing a statistically significant improvement for Yescarta in OS versus standard of care (SOC) as second-line treatment with curative intent for patients with relapsed or refractory large B-cell lymphoma (R/R LBCL) within 12 months of completion of first-line therapy.
- In May 2022, Bristol-Myers Squibb Company announced the approval of Opdivo plus Yervoy as a first line treatment for adult patients by Japan's Ministry of Health, Labour and Welfare. This may help the company to strengthen its product portfolio
Regional Analysis
Geographically, the countries covered in the market report are Singapore, Indonesia, Thailand, Malaysia, Philippines, Vietnam, Saudi Arabia, South Africa, U.A.E., Israel, Kuwait, Egypt, and rest of Middle East and Africa.
As per Data Bridge Market Research analysis:
SEA is estimated to be fastest-growing region in the MEA, Saudi Arabia and SEA CAR-T Cell Therapy Treatment Market
SEA is expected to grow due to rising adoption of advanced treatment options in the region.
For more detailed information about The MEA, Saudi Arabia and SEA CAR-T Cell Therapy Treatment Market Report, click here –