Diagnostic tests provide essential information to guide clinical decision-making, allowing healthcare professionals to accurately diagnose conditions, determine appropriate treatments, and assess the effectiveness of interventions. Rapid advancements in diagnostic technologies enhance early detection, contributing to better prognosis and improved patient outcomes. Diagnostic tests also play a pivotal role in preventive medicine, helping identify risk factors and enabling timely intervention to prevent the onset or progression of diseases. These tests are integral to public health initiatives, aiding in disease surveillance and control, especially in the context of infectious diseases and pandemics
Access full Report @ https://www.databridgemarketresearch.com/reports/north-america-diagnostic-tests-market
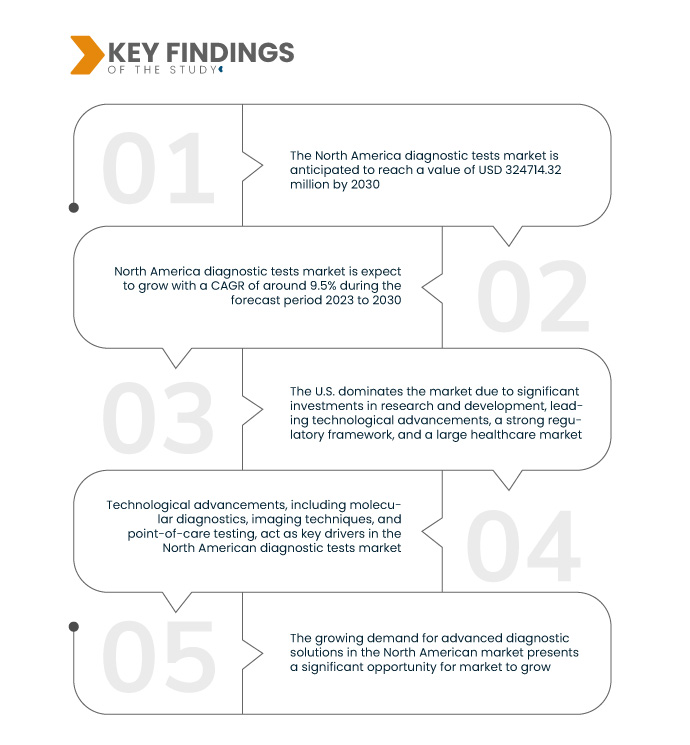
Data Bridge Market Research analyses the North America Diagnostic Tests Market, which was USD 1,57,104.45 million in 2022, and is expected to reach USD 3,24,714.32 million by 2030, at a CAGR of 9.5% during the forecast period 2023 to 2030. Significant investments in research and development in North America drive innovation in diagnostic technologies, spurring the creation of advanced and highly accurate tests.
Key Findings of the Study
Rise in a strong regulatory environment is expected to drive the market's growth rate
The North American diagnostic tests market benefits from a growing regulatory environment, spearheaded by organizations such as the FDA. This stringent regulatory framework ensures the safety and efficacy of diagnostic tests, instilling confidence among industry stakeholders. The assurance of compliance with high standards facilitates a smoother introduction of new products to the market. The FDA's oversight provides a sense of stability, encouraging innovation and investment in research and development within the sector. As a result, companies operating in the North American market are more inclined to pursue and bring forward advanced diagnostic technologies, contributing to the overall growth and dynamism of the industry.
Report Scope and Market Segmentation
Report Metric
|
Details
|
Forecast Period
|
2023 to 2030
|
Base Year
|
2022
|
Historic Years
|
2021 (Customizable to 2015-2020)
|
Quantitative Units
|
Revenue in USD Million, Volumes in Units, Pricing in USD
|
Segments Covered
|
유형(포도당 검사, 감염성 질환 검사, 세포학 검사, CBC 검사, 혈액 배양 검사, 매독 검사, 요소 검사, C 반응성 단백질 검사, 항원 검사, HBA1C 검사, 임신 검사, 지질 프로필 검사, 전해질 검사, 간 기능 검사, 대변 헬리코박터 파일로리 검사, 칼슘 검사, 교차적합 검사, 갑상선 기능 검사, 대변 현미경 검사, 소변 현미경 검사, 단위 포장 RBCS 검사, 적혈구 침윤 속도(ESR) 검사 및 기타 검사), 솔루션(서비스 및 제품), 기술(면역 측정 기반, PCR 기반, 차세대 유전자 시퀀싱, 분광법 기반, 크로마토그래피 기반, 미세유체역학, 기질 기술 및 기타), 검사 방식(처방 기반 검사 및 OTC 검사), 접근 방식(분자 진단 기기, 체외 진단 기기 및 진료소 검사 기기), 샘플 유형(소변, 타액, 혈액, 모발, 땀 및 기타), 응용 분야(심장학, 종양학, 신경학, 정형외과, 위장병학, 산부인과, 치과학 및 기타), 검사 유형(생화학, 혈액학, 미생물학, 조직병리학 및 기타), 연령(소아, 성인 및 노인), 최종 사용자(병원, 진단 센터, 연구소 및 연구소, 재택 치료, 혈액 은행, 전문 클리닉, 외래 수술 센터 및 기타), 유통 채널(직접 입찰, 소매 판매 및 온라인 판매)
|
포함 국가
|
북미의 미국, 캐나다, 멕시코
|
시장 참여자 포함
|
F-Hoffman La-Roche Ltd.(스위스), ABBOTT(미국), Danaher(미국), BD(미국), Thermo Fisher Scientific Inc.(미국), ACON Laboratories Inc.(미국), Hemosure, Inc.(미국), MicroGen Diagnostics(미국), QIAGEN(독일), Grifols, SA(스페인), BODITECH MED INC.(한국), Chembio Diagnostic Systems, Inc.(미국), Nanoentek(한국), DiaSorin SpA(이탈리아), Bio-Rad Laboratories, Inc.(미국), BIOMEDOMICS INC(미국), EKF Diagnostics Holdings plc(영국), Siemens Healthcare GmbH(독일), PerkinElmer Inc.(미국), BIOMÉRIEUX(프랑스), ARKRAY USA, Inc.(미국), Biohit Oyj(핀란드), Germaine Laboratories, Inc.(미국), Quidel Corporation(미국), Illumina, Inc.(미국), Lamdagen Corporation(미국), LifeSign LLC.(미국), Medixbiochemica(핀란드), Nova Biomedical(미국), Ortho Clinical Diagnostics(미국), Sannuo Biosensing Co., Ltd.(미국), STRECK(미국), Sysmex Corporation(일본) 등이 있습니다.
|
보고서에서 다루는 데이터 포인트
|
Data Bridge Market Research에서 큐레이팅한 시장 보고서에는 시장 가치, 성장률, 세분화, 지리적 적용 범위, 주요 기업 등 시장 시나리오에 대한 통찰력 외에도 심층적인 전문가 분석, 환자 역학, 파이프라인 분석, 가격 분석, 규제 프레임워크가 포함됩니다.
|
세그먼트 분석:
북미 진단 테스트 시장은 유형, 솔루션, 기술, 테스트 모드, 접근 방식, 샘플 유형, 응용 프로그램, 테스트 유형, 연령, 최종 사용자 및 유통 채널을 기준으로 세분화됩니다.
- 북미 진단 검사 시장은 유형별로 포도당 검사, 감염성 질환 검사, 세포학 검사, CBC 검사, 혈액 배양 검사, 매독 검사, 요소 검사, C-반응성 단백질 검사, 항원 검사, Hba1c 검사, 임신 검사, 지질 프로필 검사, 전해질 검사, 간 기능 검사, 대변 헬리코박터 파일로리 검사, 칼슘 검사, 교차 검사, 갑상선 기능 검사, 대변 현미경 검사, 소변 현미경 검사, 단위 포장 RBCS 검사, ESR 검사 및 기타 검사로 세분화됩니다.
- 솔루션을 기준으로 북미 진단 테스트 시장은 서비스와 제품으로 세분화됩니다.
- 기술에 따라 북미 진단 테스트 시장은 면역 분석 기반, PCR 기반, 차세대 유전자 시퀀싱, 분광법 기반, 크로마토그래피 기반, 미세유체, 기질 기술 및 기타로 세분화됩니다.
- 테스트 모드를 기준으로 북미 진단 테스트 시장은 처방 기반 테스트와 OTC 테스트로 구분됩니다.
- 접근 방식을 기준으로 북미 진단 테스트 시장은 분자 진단 장비, 체외 진단 장비 및 진료소 테스트 장비로 세분화됩니다.
- 샘플 유형을 기준으로 북미 진단 테스트 시장은 소변, 타액, 혈액, 머리카락, 땀 및 기타로 세분화됩니다.
- 응용 프로그램을 기준으로 북미 진단 테스트 시장은 심장학, 종양학, 신경학, 정형외과, 위장병학, 산부인과, 치과학 및 기타로 세분화됩니다.
- 테스트 유형을 기준으로 북미 진단 테스트 시장은 생화학, 혈액학, 미생물학, 조직병리학 및 기타로 세분화됩니다.
- 연령을 기준으로 북미 진단 테스트 시장은 소아, 성인 및 노인으로 구분됩니다.
- 최종 사용자를 기준으로 북미 진단 테스트 시장은 병원, 진단 센터, 연구실 및 연구소, 연구 기관, 재택 치료, 혈액 은행, 전문 클리닉, 외래 수술 센터 등으로 세분화됩니다.
- 유통 채널을 기준으로 북미 진단 테스트 시장은 직접 입찰, 소매 판매 및 온라인 판매로 세분화됩니다.
주요 플레이어
Data Bridge Market Research에서는 북미 진단 검사 시장의 주요 기업으로 F-Hoffman La-Roche Ltd.(스위스), ABBOTT(미국), Danaher(미국), BD(미국), Thermo Fisher Scientific Inc.(미국), ACON Laboratories Inc.(미국), Hemosure, Inc.(미국)를 꼽았습니다.
시장 개발
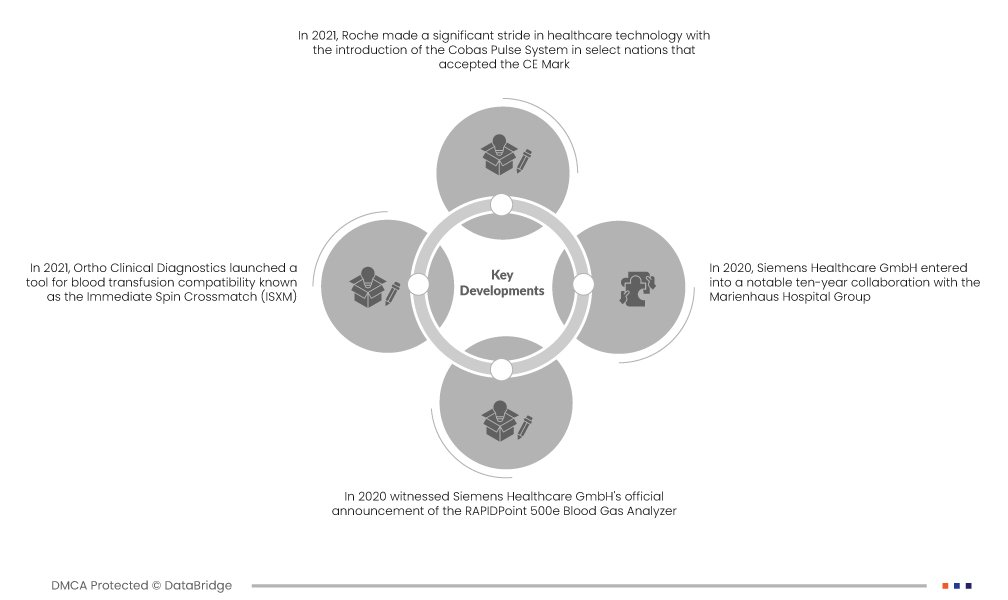
- 2021년, 로슈는 CE 마크를 획득한 일부 국가에 코바스 펄스 시스템(Cobas Pulse System)을 출시하며 의료 기술 분야에서 큰 진전을 이루었습니다. 로슈 진단의 최신 세대 제품인 이 혁신적인 현장 진단(Point of Care) 솔루션은 전문 혈당 관리 분야에서 괄목할 만한 발전을 이루었습니다. 네트워크 연결을 지원하도록 설계된 이 시스템은 진단 분야에서 최첨단 솔루션을 제공하려는 로슈의 의지를 보여주었습니다.
- 2021년, 오쏘 클리니컬 다이어그노스틱스(Ortho Clinical Diagnostics)는 수혈 적합성 검사 도구인 Immediate Spin Crossmatch(ISXM)를 출시했습니다. 이 도구는 오쏘 비전(Ortho Vision)과 오쏘 비전 맥스(Ortho Vision Max) 분석기에 통합되었습니다. ISXM의 도입은 수혈 시 수혈자와 헌혈자 부적합을 빠르고 정확하게 식별할 수 있게 해 주는 이 분야의 중추적인 발전이었습니다. 이는 진단 역량 향상과 수혈 절차의 안전성 및 효능 확보에 대한 오쏘 클리니컬 다이어그노스틱스의 헌신을 보여주는 사례였습니다.
- 2020년, 지멘스 헬스케어(Siemens Healthcare GmbH)는 RAPIDPoint 500e 혈액 가스 분석기를 공식 발표했습니다. 이는 지멘스의 제품 포트폴리오를 크게 확장하는 계기가 되었으며, 특히 COVID-19 팬데믹에 맞서기 위한 전 세계적인 노력의 맥락에서 더욱 광범위한 의미를 지녔습니다. RAPIDPoint 500e는 혈액 가스 분석에 중요한 역할을 수행하여 COVID-19 사례의 포괄적인 관리에 기여했습니다. 이번 출시는 지멘스의 기술 혁신에 대한 헌신을 보여주는 동시에, 의료 분야 과제 해결의 핵심 기업으로서의 입지를 굳건히 다지는 계기가 되었습니다.
- 2020년, 지멘스 헬스케어(Siemens Healthcare GmbH)는 마리엔하우스 병원 그룹(Marienhaus Hospital Group)과 10년간의 주목할 만한 협력 계약을 체결했습니다. 이 전략적 파트너십은 지멘스, 특히 진단 분야에서 지멘스에 지대한 영향을 미쳤습니다. 이 협력은 지멘스 헬스케어에 장기적인 이익을 가져다주어 진단 분야에서의 입지를 강화할 것으로 예상되었습니다. 진단 분야를 넘어, 이 계약은 회사의 재무 상태에도 긍정적인 영향을 미칠 것으로 예상되며, 이는 지멘스가 회사의 전반적인 성장과 성공에 기여하는 파트너십을 육성하려는 적극적인 자세를 보여주는 좋은 예입니다.
지역 분석
지리적으로 북미 진단 테스트 시장 보고서에서 다루는 국가는 북미의 미국, 캐나다, 멕시코입니다.
Data Bridge Market Research 분석에 따르면:
미국은 2023-2030년 예측 기간 동안 북미 진단 테스트 시장 에서 지배적인 지역입니다 .
미국은 여러 요인이 복합적으로 작용하여 북미 진단 검사 시장을 장악하고 있습니다. 막대한 연구개발 투자, 기술 발전의 선두주자, FDA가 주도하는 강력한 규제 체계, 그리고 광범위하고 다양한 의료 시장이 미국의 시장 리더십에 기여하고 있습니다. 학계와 산업계의 협력, 개인 맞춤 의료에 대한 강조, 그리고 주요 제약 및 생명공학 기업의 입지는 미국의 시장 영향력을 더욱 강화하고 있습니다.
북미 진단 테스트 시장 보고서 에 대한 자세한 내용은 여기를 클릭하세요 - https://www.databridgemarketresearch.com/reports/north-america-diagnostic-tests-market