Clinical laboratory services play a pivotal role in preventive medicine by conducting screenings and tests that enable the early detection of diseases. These laboratories identify potential risk factors through assessments such as cholesterol levels, blood pressure measurements, and cancer screenings, empowering healthcare providers to intervene promptly. Early detection allows for timely medical interventions and the implementation of preventive measures, ultimately mitigating the progression of diseases and minimizing the likelihood of severe health outcomes.
Access Full Report @ https://www.databridgemarketresearch.com/reports/philippines-clinical-laboratory-services-market
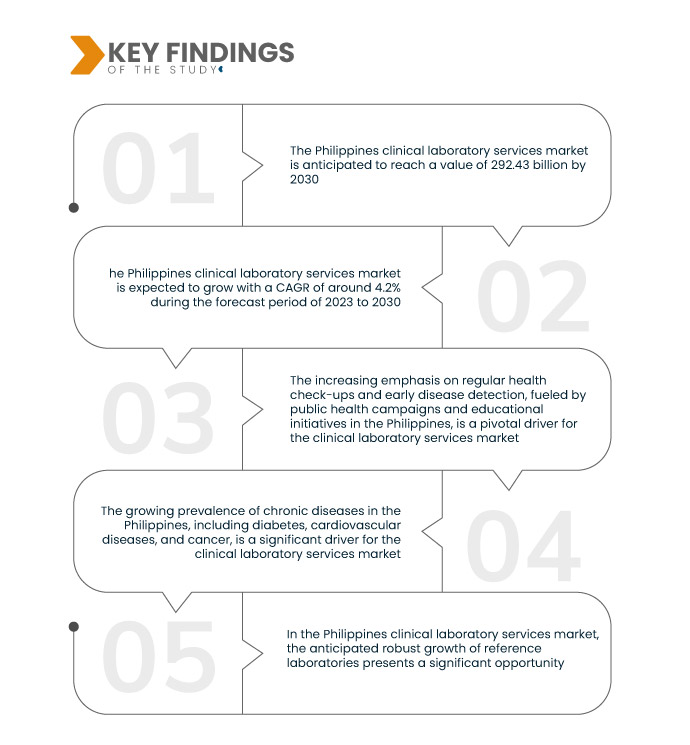
Data Bridge Market Research analyses the Philippines Clinical Laboratory Services Market, which is USD 210.42 billion in 2022 and is expected to reach USD 292.43 billion by 2030, at a CAGR of 4.2% during the forecast period 2023 to 2030. In the Philippines, government initiatives promoting supportive policies, regulations, and healthcare infrastructure improvements, including active engagement in public-private partnerships and increased investments, serve as crucial drivers for the growth of the clinical laboratory services market.
Key Findings of the Study
Increasing evolution of diagnostic technologies is expected to drive the market's growth rate
In the Philippines clinical laboratory services market, the constant evolution of diagnostic technologies serves as a pivotal driver. Ongoing advancements in diagnostic methods offer clinical laboratories the ability to provide newer and more accurate testing options. This attracts healthcare providers seeking precise diagnostic tools and resonates with patients, fostering increased confidence in the reliability of laboratory services. Adopting state-of-the-art diagnostic technologies enhances the capabilities of clinical laboratories, positioning them as crucial contributors to effective healthcare delivery. This driver underscores the market's growth as it aligns with the demand for advanced and sophisticated diagnostic solutions in the evolving healthcare landscape of the Philippines.
Report Scope and Market Segmentation
|
Report Metric
|
Details
|
|
Forecast Period
|
2023 to 2030
|
|
Base Year
|
2022
|
|
Historic Years
|
2021 (Customizable to 2015-2020)
|
|
Quantitative Units
|
Revenue in USD Billion, Volumes in Units, Pricing in USD
|
|
Segments Covered
|
Specialty (Clinical Chemistry Testing, Hematology Testing, Microbiology Testing, Immunology Testing, Drugs of Abuse Testing, Cytology Testing and Genetic Testing), Provider (Independent and Reference Laboratories, Hospital-Based Laboratories and Nursing and Physician Office-Based Laboratories), Application (Drug Discovery Related Services, Drug Development Related Services, Bioanalytical and Lab Chemistry Services, Toxicology Testing Services, Cell and Gene Therapy Related Services, Preclinical and Clinical Trial Related Services and Other Clinical Laboratory Services), Service Type (Routine Testing Services, Esoteric Services And Anatomic Pathology Services)
|
|
Market Players Covered
|
Abbott (U.S.), ARUP Laboratories (U.S.), OPKO Health, Inc. (U.S.), ioscientia Healthcare GmbH (Germany), Charles River Laboratories (U.S.), NeoGenomics Laboratories (U.S.), Genoptix, Inc. (U.S.), Healthscope (Australia), The Laboratory Glassware Co. (U.S.), Laboratory Corporation of America Holdings (U.S.), Fresenius Medical Care AG and Co. KGaA (Germany), QIAGEN (Germany), Quest Diagnostics Incorporated (U.S.), Siemens Healthcare Private Limited (Germany), Tulip Diagnostics (P) Ltd. (India), Sonic Healthcare Limited (Australia), Merck KGaA (Germany)
|
|
Data Points Covered in the Report
|
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
|
Segment Analysis:
The Philippines clinical laboratory services market is segmented on the basis of specialty, provider, application, and service type.
- On the basis of speciality, the Philippines clinical laboratory services market is segmented into clinical chemistry testing, hematology testing, microbiology testing, immunology testing, drugs of abuse testing, cytology testing, and genetic testing
- On the basis of provider, the Philippines clinical laboratory services market is segmented into independent and reference laboratories, hospital-based laboratories, and nursing and physician office-based laboratories
- On the basis of application, the Philippines clinical laboratory services market is segmented into drug discovery related services, drug development related services, bioanalytical and lab chemistry services, toxicology testing services, cell and gene therapy related services, preclinical and clinical trial related services, and other clinical laboratory services
- On the basis of service type, the Philippines clinical laboratory services market is segmented into routine testing services, esoteric services, and anatomic pathology services
Major Players
Data Bridge Market Research recognizes the following companies as the major Philippines clinical laboratory services market players in Philippines clinical laboratory services market are Abbott (U.S.), ARUP Laboratories (U.S.), OPKO Health, Inc. (U.S.), ioscientia Healthcare GmbH (Germany), Charles River Laboratories (U.S.), NeoGenomics Laboratories (U.S.), Genoptix, Inc. (U.S.), Healthscope (Australia), The Laboratory Glassware Co. (U.S.).
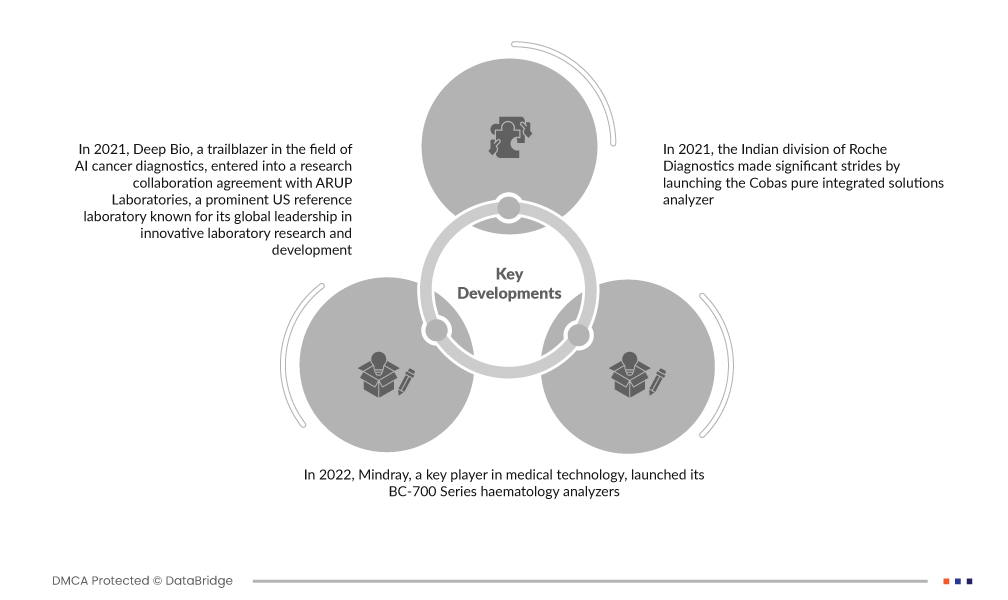
Market Developments
- In 2021, Deep Bio, a trailblazer in the field of AI cancer diagnostics, entered into a research collaboration agreement with ARUP Laboratories, a prominent U.S. reference laboratory known for its global leadership in innovative laboratory research and development. This strategic collaboration aimed to leverage artificial intelligence to advance cancer diagnostics. Through joining forces with ARUP Laboratories, Deep Bio sought to enhance its capabilities in utilizing AI technologies for more accurate and efficient cancer diagnosis
- In 2022, Mindray, a key player in medical technology, launched its BC-700 Series haematology analyzers. Specifically designed for small-to-medium-sized laboratories, these analyzers offered a comprehensive range of tests, including complete blood count (CBC) and erythrocyte sedimentation rate (ESR) tests. The launch of the BC-700 Series represented Mindray's commitment to providing state-of-the-art solutions to laboratories, contributing to improved efficiency and accuracy in haematological diagnostics
- In 2021, the Indian division of Roche Diagnostics made significant strides by launching the Cobas pure integrated solutions analyzer. This advanced diagnostic system featured a more intelligent design, catering specifically to the country's vast number of laboratories and hospitals. Roche Diagnostics aimed to address the unique challenges faced by the Indian healthcare landscape by providing a sophisticated and user-friendly solution. The Cobas pure integrated solutions analyzer exemplified Roche's commitment to delivering cutting-edge technologies tailored to the needs of diverse healthcare settings
For more detailed information about the Philippines clinical laboratory services market report, click here – https://www.databridgemarketresearch.com/reports/philippines-clinical-laboratory-services-market