The osteoporosis drugs market has witnessed significant advancements in recent years. Notably, the development of novel medications, including bisphosphonates, monoclonal antibodies, and selective estrogen receptor modulators, has improved treatment options. These drugs enhance bone density and reduce fracture risks, addressing the core concern of osteoporosis. Moreover, patient-friendly features such as extended dosing intervals and reduced side effects have increased adherence to treatment regimens. These advancements in drug therapies are fostering improved patient outcomes and quality of life for those affected by osteoporosis.
Access Full Report @ https://www.databridgemarketresearch.com/reports/saudi-arabia-osteoporosis-drug-market
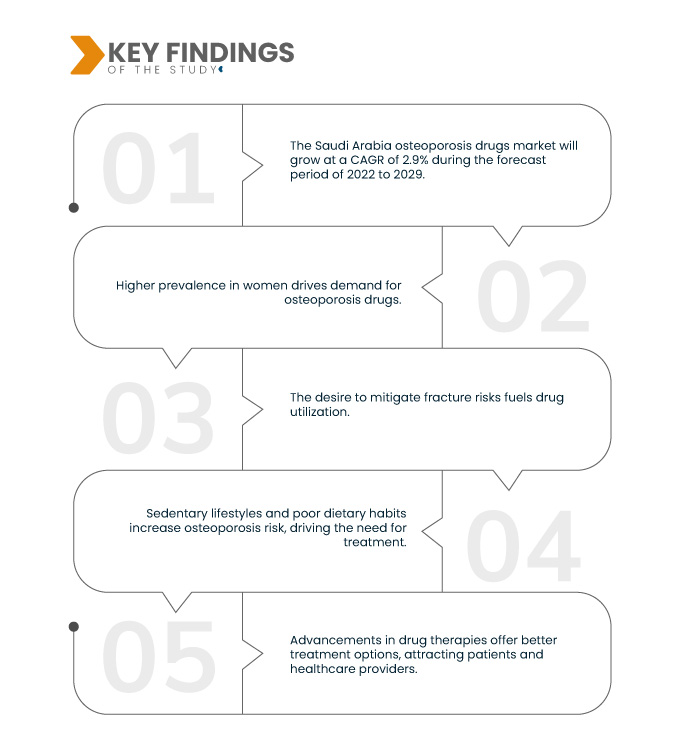
Data Bridge Market Research analyses that the Saudi Arabia Osteoporosis Drugs Market will grow at a CAGR of 2.9% during the forecast period of 2022 to 2029. As the elderly population grows, so does the susceptibility to osteoporosis, a condition characterized by brittle bones. This demographic shift increases the demand for osteoporosis drugs to prevent fractures and maintain bone health, driving the market for such medications.
Key Findings of the Study
Government initiatives are expected to drive the market's growth rate
Government initiatives play a crucial role in addressing osteoporosis. They often involve public health campaigns to raise awareness, promote bone health, and encourage early diagnosis. Some governments may subsidize osteoporosis medications or offer reimbursement programs to make treatment more accessible and affordable. Additionally, regulatory agencies set guidelines for osteoporosis management and drug approvals, ensuring safety and efficacy. These initiatives collectively aim to reduce the burden of osteoporosis, improve patient outcomes, and lower healthcare costs.
Report Scope and Market Segmentation
|
Report Metric
|
Details
|
|
Forecast Period
|
2022 to 2029
|
|
Base Year
|
2021
|
|
Historic Years
|
2020 (Customizable to 2014-2019)
|
|
Segments Covered
|
Type (Primary Osteoporosis and Secondary Osteoporosis), Drugs (Bisphosphonates, Monoclonal Antibodies, Hormone Therapy and Others), Drugs Type (Branded and Generic), Route of Administration (Injectable, Oral and Others), Gender (Female and Male), Age Group (Geriatrics, Adults and Pediatrics), End User (Hospitals, Specialty Clinics, Homecare and Others), Distribution Channel (Direct Tender, Retail Sales and Others)
|
|
Countries Covered
|
Spain
|
|
Market Players Covered
|
Eli Lilly and Company (U.S.), Novartis AG (Switzerland), Amgen Inc. (U.S.), Tabuk Pharmaceuticals (Saudi Arabia), SPIMACO (Saudi Arabia), Hikma Pharmaceuticals PLC (Jordan), Pfizer Inc. (U.S.), Merck KGaA (Germany), Apotex Inc. (Canada), ABIOGEN PHARMA S.p.A. (Italy), Sudair Pharma (Saudi Arabia), tadawipharma. (Saudi Arabia), Fresenius SE and Co. KGaA (Germany), Hayat Pharmaceutical Industries Co. PLC (Jordan), SAJA Pharmaceuticals (Saudi Arabia)
|
|
Data Points Covered in the Report
|
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
|
Segment Analysis:
The Saudi Arabia osteoporosis drugs market is categorized into eight notable segments based on type, drugs, drugs type, route of administration, gender, age group, end user, and distribution channel.
- On the basis of type, Saudi Arabia osteoporosis drugs market is segmented into primary osteoporosis and secondary osteoporosis.
- On the basis of drugs, Saudi Arabia osteoporosis drugs market is segmented into bisphosphonates, monoclonal antibodies, hormone therapy, and others.
- On the basis of drugs type, Saudi Arabia osteoporosis drugs market is segmented into branded and generic.
- On the basis of route of administration, Saudi Arabia osteoporosis drugs market is segmented into injectable, oral, and others.
- On the basis of gender, Saudi Arabia osteoporosis drugs market is segmented into female and male.
- On the basis of age group, Saudi Arabia osteoporosis drugs market is segmented into geriatrics, adults, and pediatrics.
- On the basis of end user, Saudi Arabia osteoporosis drugs market is segmented into hospitals, specialty clinics, homecare, and others.
- On the basis of distribution channel, Saudi Arabia osteoporosis drugs market is segmented into direct tender, retail sales, and others.
Major Players
Data Bridge Market Research recognizes the following companies as the Saudi Arabia osteoporosis drugs market players in Saudi Arabia osteoporosis drugs market are Eli Lilly and Company (U.S.), Novartis AG (Switzerland), Amgen Inc. (U.S.), Tabuk Pharmaceuticals (Saudi Arabia), SPIMACO (Saudi Arabia), Hikma Pharmaceuticals PLC (Jordan), Pfizer Inc. (U.S.), Merck KGaA (Germany), Apotex Inc. (Canada).
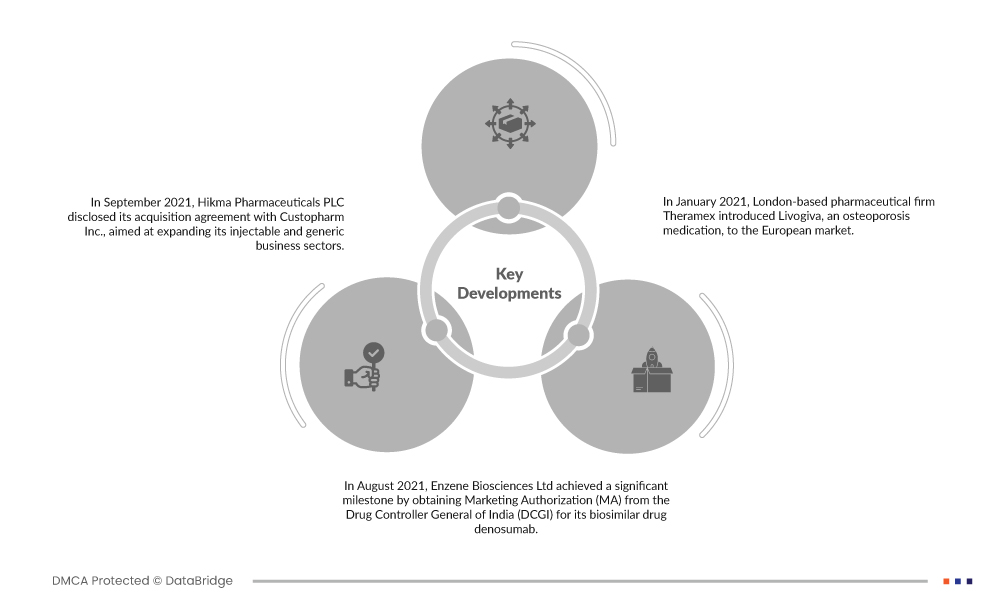
Market Developments
- In September 2021, Hikma Pharmaceuticals PLC disclosed its acquisition agreement with Custopharm Inc., aimed at expanding its injectable and generic business sectors. Custopharm held an impressive track record, securing 13 US FDA approvals, including four pioneering Abbreviated New Drug Application (ANDA) approvals, one of which received Competitive Generic Therapy (CGT) designation. Additionally, they obtained a novel 505(b)(2) NDA approval. This strategic acquisition bolstered Hikma's product portfolio and contributed to the overall revenue growth of the company.
- In August 2021, Enzene Biosciences Ltd achieved a significant milestone by obtaining Marketing Authorization (MA) from the Drug Controller General of India (DCGI) for its biosimilar drug denosumab. This approval signifies that the drug is authorized for marketing and use in India. Denosumab is indicated for the treatment of osteoporosis in adult patients. This regulatory approval underscores the company's commitment to providing effective treatments for osteoporosis and represents a positive step in addressing this medical condition in India.
- In January 2021, London-based pharmaceutical firm Theramex introduced Livogiva, an osteoporosis medication, to the European market. This launch marked an important addition to the company's portfolio of products aimed at addressing bone health. Livogiva is designed to provide effective treatment for individuals dealing with osteoporosis, contributing to improved bone density and overall health. The introduction of this medication exemplifies Theramex's commitment to enhancing the well-being of patients with bone-related conditions in Europe.
For more detailed information about the Saudi Arabia osteoporosis drugs market report, click here – https://www.databridgemarketresearch.com/reports/saudi-arabia-osteoporosis-drug-market