The prevalence of diseases and conditions such as cardiovascular disease (CVD’s), cancer, stroke, diabetes, and respiratory diseases has increased significantly over a period of time.
This increase in prevalence of chronic diseases can be attributed to unhealthy lifestyles, including excess consumption of alcohol and tobacco. The above-mentioned factors also increase health risks such as obesity, high blood pressure, and cholesterol, which in turn contribute towards the unprecedented rise in the cases of chronic diseases. With increasing prevalence of diseases, the demand for early diagnosis is also increasing.
With the increasing prevalence of chronic diseases, the demand for development and innovation of diagnostic technique is also increasing. This increasing demand allows companies to focus on introducing various diagnosis and treatments in the market in order to meet the rising demand for effective diagnostic.
Access full Report @ https://www.databridgemarketresearch.com/reports/us-clinical-chemistry-analyzer-market
Data Bridge Market Research analyzes that the U.S. Clinical Chemistry Analyzer Market is expected to grow at a CAGR of 3.9% in the forecast period of 2024 to 2031 and is expected to reach USD 7.20 billion by 2031 from 5.40 billion in 2023. The reagents segment is projected to propel the market growth due to the rising demand for better diagnostic and treatment options.
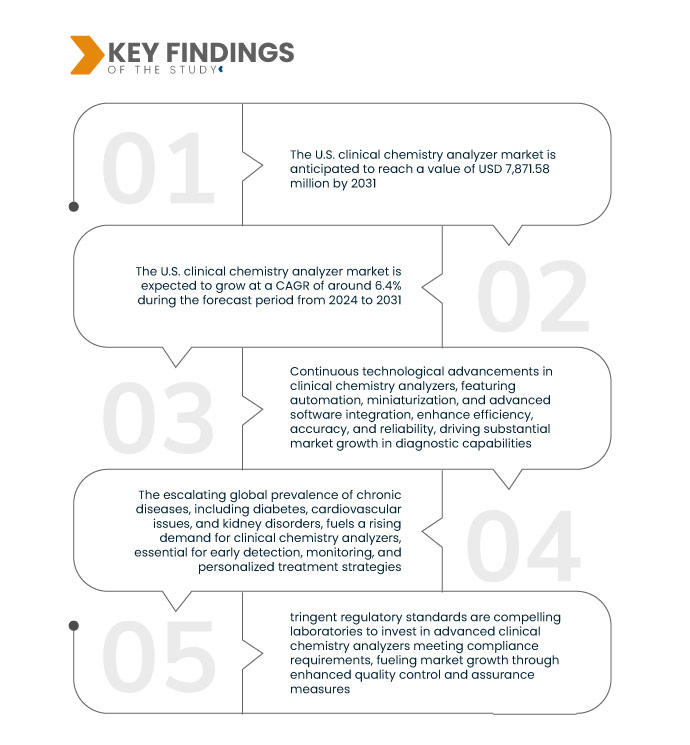
Key Findings of the Study
Development in Database Management Tools and Wide Acceptance of Point-Of-Care (Poc) Testing Solutions
Report Scope and Market Segmentation
|
Report Metric
|
Details
|
|
Forecast Period
|
2024 to 2031
|
|
Base Year
|
2023
|
|
Historic Year
|
2022 (Customizable to 2016–2021)
|
|
Quantitative Units
|
Revenue in USD Million, and Pricing in USD
|
|
Segments Covered
|
Product Type (Analyzers, and Reagents), Test Type (Metabolic Panel (BMP), General Chemistry Test, Liver Panel, Electrolyte Panel, Renal Profile, Lipid Profile, Specialty Chemical Tests, Thyroid Function Panel, and Others), Modality (Benchtop, Standalone, Portable, and Others), Application (Inflammatory Diseases, Diabetes, Cancer, Abuse of Drugs, Renal Failure, Cardiovascular Diseases, Nutritional Deficiency, and Others), Specimen (Serum, Plasma, Urine, Cerebrospinal Fluid (CSF), Amniotic Fluid, Saliva, Blood, and Others), End Users (Independent Diagnostic Laboratories (Laboratories Outside Hospitals), and Hospitals), Distribution Channel (Direct Tender, Retail Sales, and Others)
|
|
Countries Covered
|
U.S.
|
|
Market Players Covered
|
F. Hoffmann-La Roche Ltd (Switzerland), Beckman Coulter, Inc. (Subsidiary of Danaher) (U.S.), Thermo Fisher Scientific Inc. (U.S.), Abbott (U.S.), Shenzhen Mindray Bio-Medical Electronics Co., Ltd. (China), HORIBA (Japan), ELITech Group (France), Randox Laboratories Ltd. (U.S.), Medica Corporation (U.S.), JEOL Ltd. (U.S.), FURUNO ELECTRIC CO.,LTD. (Japan) and Hitachi High-Tech Corporation (Japan), among others
|
|
Data Points Covered in the Report
|
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, geographically represented company-wise production and capacity, network layouts of distributors and partners, detailed and updated price trend analysis and deficit analysis of supply chain and demand
|
Segment Analysis
U.S. clinical chemistry analyzer market is segmented into seven notable segments which are based on product type, test type, modality, application, specimen, end users and distribution channel.
- Based on product type, the market is segmented into into analyzers and reagents
In 2024, the reagents segment of the product type segment is expected to dominate the market
In 2024, the reagents segment is expected to dominate the market with a market share of 78.58% due to the rising demand for new diagnostic options.
- Based on test type, the market is segmented into metabolic panel (BMP), general chemistry test, liver panel, electrolyte panel, renal profile, lipid profile, specialty chemical tests, thyroid function panel and others
In 2024, the metabolic panel (BMP) segment of the test type segment is expected to dominate the market
In 2024, the metabolic panel (BMP) is expected to dominate the market with a market share of 29.28% due to the growing emphasis on preventive healthcare and wellness.
- Based on modality, the market is segmented into benchtop, standalone, portable and others. In 2024, the benchtop segment is expected to dominate the market with a market share of 67.12%
- Based on application, the market is segmented into inflammatory & infectious diseases, diabetes, cancer, abuse of drugs, renal failure, cardiovascular diseases, nutritional deficiency and others. In 2024, the inflammatory & infectious diseases segment is expected to dominate the market with a market share of 31.81%
- Based on specimen, the market is segmented into serum plasma, urine, cerebrospinal fluid (CSF), amniotic fluid, saliva, blood and others. In 2024, the serum segment is expected to dominate the market with a market share of 33.79%
- Based on end user, the market is segmented into independent diagnostic laboratories (laboratories outside hospitals) and hospitals. In 2024, the hospitals segment is expected to dominate the market with a market share of 53.21%
- Based on distribution channel, the market is segmented into direct tender, retail sales and others. In 2024, the direct tender segment is expected to dominate the market with a market share of 49.33%
Major Players
Data Bridge Market Research analyzes F. Hoffmann-La Roche Ltd (Switzerland), Beckman Coulter, Inc. (Subsidiary of Danaher) (U.S.), Thermo Fisher Scientific Inc. (U.S.), Abbott (U.S.), Shenzhen Mindray Bio-Medical Electronics Co., Ltd. (China) as the major player in this market.
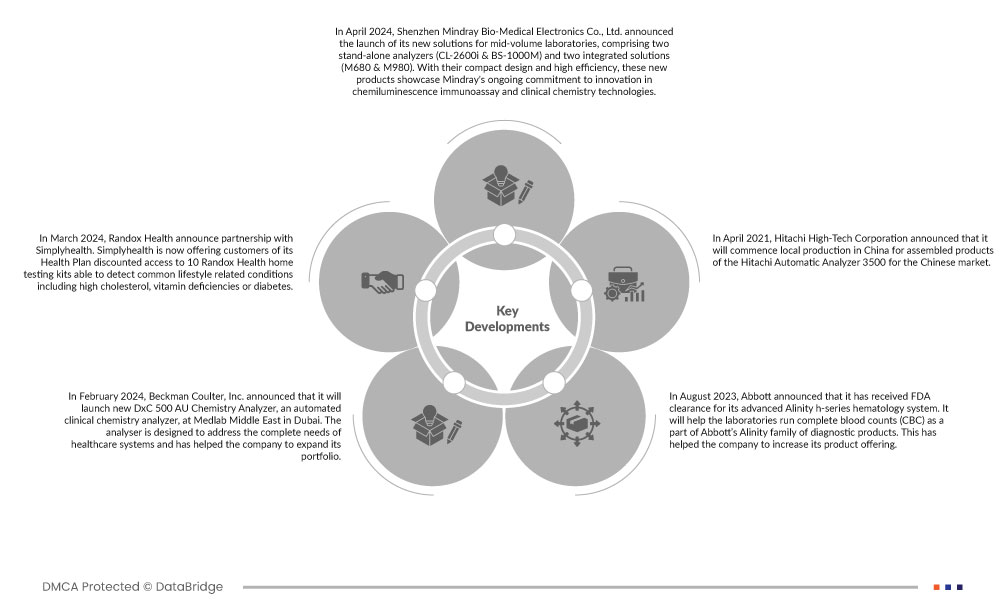
Market Development
- In April 2024, Shenzhen Mindray Bio-Medical Electronics Co., Ltd. announced the launch of its new solutions for mid-volume laboratories, comprising two stand-alone analyzers (CL-2600i & BS-1000M) and two integrated solutions (M680 & M980). With their compact design and high efficiency, these new products showcase Mindray's ongoing commitment to innovation in chemiluminescence immunoassay and clinical chemistry technologies.
- In March 2024, Randox Health announce partnership with Simplyhealth. Simplyhealth is now offering customers of its Health Plan discounted access to 10 Randox Health home testing kits able to detect common lifestyle related conditions including high cholesterol, vitamin deficiencies or diabetes.
- In February 2024, Beckman Coulter, Inc. announced that it will launch new DxC 500 AU Chemistry Analyzer, an automated clinical chemistry analyzer, at Medlab Middle East in Dubai. The analyser is designed to address the complete needs of healthcare systems and has helped the company to expand its portfolio.
- In July 2022, Thermo Fisher Scientific Inc. showcased its innovative diagnostic technologies, assays and a complement of solutions for researchers developing new diagnostics at 74th American Association for Clinical Chemistry Annual Scientific Meeting and Clinical Laboratory Exposition (AACC 2022). This has helped the company to display its innovative products and new technologies in the market.
- In April 2021, Hitachi High-Tech Corporation announced that it will commence local production in China for assembled products of the Hitachi Automatic Analyzer 3500 for the Chinese market. Hitachi High-tech has been producing the small 3110 model clinical analyzers locally since 2018, but by adding the new 3500 model to our lineup, we will be able to meet growing demand in the larger market for medium-sized automatic clinical analyzer.
Regional Analysis
Geographically, the country covered in the clinical chemistry analyzer market report is U.S.
For more detailed information about the U.S. Clinical Chemistry Analyzer Market report, click here – https://www.databridgemarketresearch.com/reports/us-clinical-chemistry-analyzer-market