내시경 재처리는 의료 환경에서 교차 오염을 막는 최전선 방어 수단으로 필수적입니다. 매일 여러 차례의 시술이 수행되므로 환자 간 감염 전파 위험은 항상 우려되는 문제입니다. 내시경의 꼼꼼한 세척 및 소독을 포함하는 강력한 재처리 프로토콜은 잠재적 감염의 사슬을 끊는 데 필수적입니다. 멸균 내시경 환경을 유지하기 위한 노력은 개별 환자를 보호하고 의료 안전 및 질 향상이라는 더 큰 목표에 크게 기여합니다.
전체 보고서는 https://www.databridgemarketresearch.com/reports/us-endoscope-reprocessing-market 에서 확인하세요.
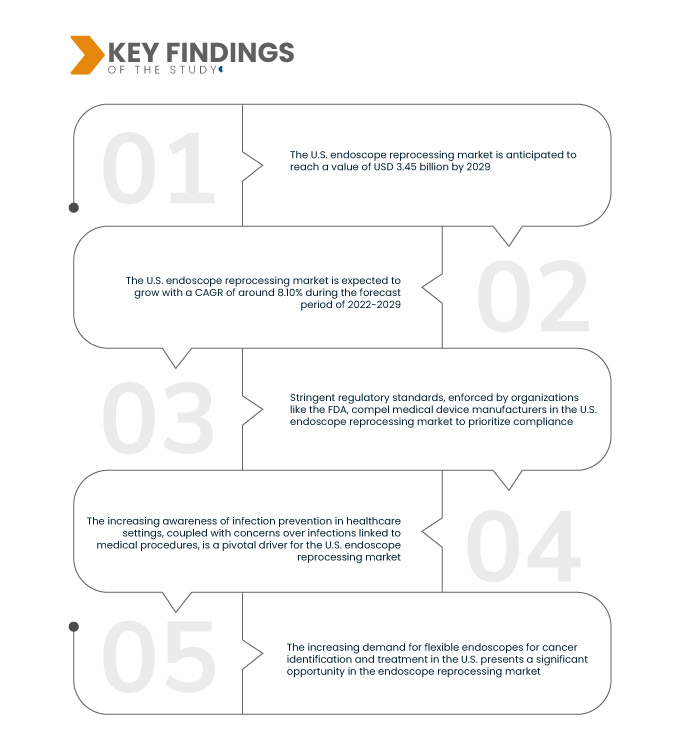
Data Bridge Market Research는 2021년에 18억 5천만 달러였던 미국 내시경 재처리 시장을 분석했습니다 . 이 시장은 2029년까지 34억 5천만 달러로 성장할 것으로 예상되며, 2022년부터 2029년까지 예측 기간 동안 연평균 성장률 8.10%를 기록할 것으로 예상됩니다. 최소 침습적 특성으로 인해 미국에서 내시경 시술에 대한 수요가 급증함에 따라 의료 서비스 제공업체는 진단 및 치료를 위해 이러한 기술을 도입하게 되었습니다.
연구의 주요 결과
미국의 의료비 지출 증가로 시장 성장률이 높아질 것으로 예상됩니다.
미국의 의료비 지출 증가는 내시경 재처리 시장의 핵심 동력입니다. 의료 부문에 대한 재정 지원 확대는 의료 기관들이 최첨단 의료 장비, 특히 첨단 내시경 재처리 시스템에 투자할 수 있도록 지원합니다. 이러한 자금 지원 급증은 감염 예방과 환자 안전에 대한 의지를 보여줍니다. 상당한 자원을 기꺼이 투입하려는 의지를 바탕으로 의료 기관들은 최첨단 재처리 기술을 도입하여 시장 확대를 촉진하고 있습니다.
보고서 범위 및 시장 세분화
보고서 메트릭
|
세부
|
예측 기간
|
2022년부터 2029년까지
|
기준 연도
|
2021
|
역사적인 해
|
2020 (2014-2019년으로 맞춤 설정 가능)
|
양적 단위
|
매출(10억 달러), 볼륨(단위), 가격(USD)
|
다루는 세그먼트
|
제품(장비 및 소모품), 방식(벤치탑 내시경 재처리기 및 독립형 내시경 재처리기), 프로세스 유형(자동 세척 소독제 및 수동 세척 솔루션), 최종 사용자(병원, 외래 수술 센터 (ASCS), 진료소, 진단 센터 및 기타)
|
시장 참여자 포함
|
Cantel Medical(미국), ASP(미국), OlympU.S. Corporation(일본), Ecolab(미국), STERIS(아일랜드), Getinge AB(스웨덴), Wassenburg Medical(네덜란드), CONMED Corporation(미국), Belimed AG(스위스), Endo-Technik W. Griesat(독일), CU.S.tom Ultrasonics.com(미국), Steelco SpA(이탈리아), BES Rehab Ltd(영국), ARC Group of Companies Inc.(캐나다), Metrex Research, LLC. (캐나다), Richard Wolf GmbH(독일), MEDALKAN(그리스), Micro-Scientific, LLC(미국), Borer Chemie AG(스위스), Tuttnauer(네덜란드), ATMS(캐나다), Summit Imaging, Inc.(미국), Medonica Co. LTD(한국), SHINVA MEDICAL INSTRUMENT CO. LTD(중국), Medical Devices Group Srl(이탈리아)
|
보고서에서 다루는 데이터 포인트
|
Data Bridge Market Research에서 큐레이팅한 시장 보고서에는 시장 가치, 성장률, 세분화, 지리적 적용 범위, 주요 기업 등 시장 시나리오에 대한 통찰력 외에도 심층적인 전문가 분석, 환자 역학, 파이프라인 분석, 가격 분석, 규제 프레임워크가 포함됩니다.
|
세그먼트 분석:
미국 내시경 재처리 시장은 제품, 방식, 공정 유형 및 최종 사용자를 기준으로 세분화됩니다.
- 미국 내시경 재처리 시장은 제품을 기준으로 장비와 소모품으로 구분됩니다.
- 모달리티를 기준으로 미국 내시경 재처리 시장은 벤치탑 내시경 재처리기와 독립형 내시경 재처리기로 구분됩니다.
- 미국 내시경 재처리 시장은 프로세스 유형을 기준으로 자동 세척 소독제와 수동 세척 솔루션으로 구분됩니다.
- 최종 사용자를 기준으로 미국 내시경 재처리 시장은 병원, 외래 수술 센터(ASCS), 진료소, 진단 센터 및 기타로 세분화됩니다.
주요 플레이어
Data Bridge Market Research는 미국 내시경 재처리 시장의 주요 미국 내시경 재처리 시장 참여자로 다음 회사를 인식합니다. Cantel Medical(미국), ASP(미국), OlympU.S. Corporation(일본), Ecolab(미국), STERIS(아일랜드), Getinge AB(스웨덴), Wassenburg Medical(네덜란드), CONMED Corporation(미국), Belimed AG(스위스), Endo-Technik W. Griesat(독일)
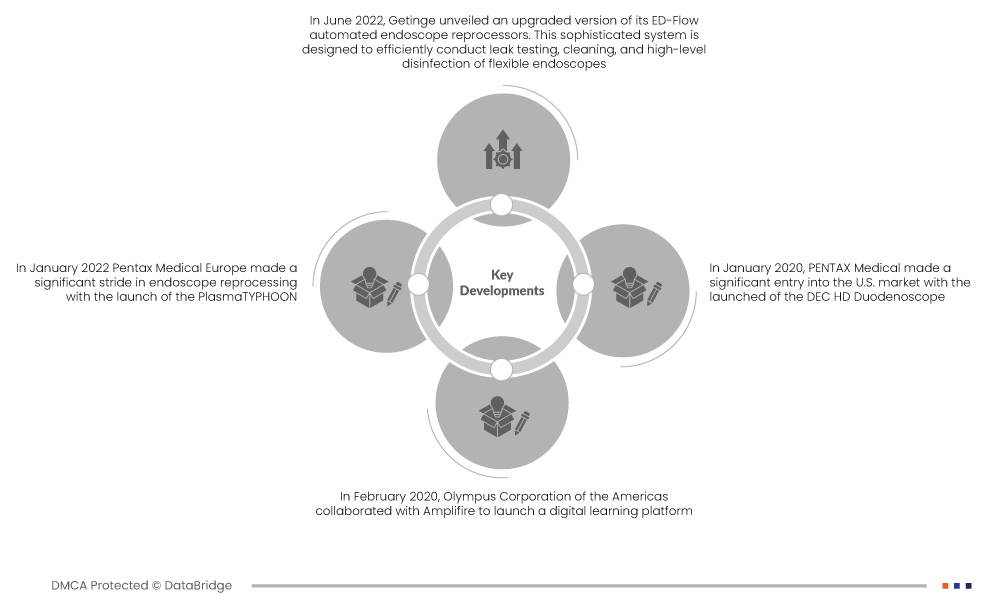
시장 개발
- 2022년 6월, 게팅게(Getinge)는 ED-Flow 자동 내시경 세척기의 업그레이드 버전을 출시했습니다. 이 정교한 시스템은 연성 내시경의 누출 검사, 세척 및 고수준 소독을 효율적으로 수행하도록 설계되었습니다. 효과적이고 신뢰할 수 있는 결과 제공에 중점을 둔 이 혁신은 의료 장비 위생의 높은 기준을 유지하는 데 기여합니다.
- 2022년 1월, 펜탁스 메디컬 유럽(Pentax Medical Europe)은 PlasmaTYPHOON을 출시하며 내시경 재처리 분야에서 큰 진전을 이루었습니다. 이 최첨단 솔루션은 초고속 기능을 자랑하며 내시경 건조 및 보관에 혁신을 가져올 것입니다. 이 장치는 생산성과 추적성을 향상시킬 뿐만 아니라 환자 안전을 강화하기 위한 내시경 재처리 발전에 중추적인 역할을 합니다.
- 2020년 2월, 올림푸스 아메리카스(Olympus Corporation of the Americas)는 앰플리파이어(Amplifire)와 협력하여 디지털 학습 플랫폼을 출시했습니다. 이 플랫폼은 내시경 재처리 기술자를 위해 특별히 제작되어 새롭고 효율적인 학습 방식을 제공합니다. 이 프로젝트는 내시경 역행성 담췌관조영술(ERCP)에 사용되는 복잡한 의료용 내시경인 올림푸스 TJF-Q180V 십이지장경의 재처리를 중심으로 진행됩니다.
- 2020년 1월, 펜탁스 메디컬(PENTAX Medical)은 DEC HD 십이지장경(DEC HD Duodenoscope) 출시로 미국 시장에 본격적으로 진출했습니다. 이 첨단 의료 기기는 고화질 기능을 갖추고 있으며, 다양한 일회용 부품을 내장하여 내시경 재처리 과정을 개선했습니다. 특히 멸균 원위부 캡과 엘리베이터 레버는 내시경 재처리 과정의 위생 관리 강화를 위한 펜탁스의 노력을 보여줍니다.
미국 내시경 재처리 시장 보고서 에 대한 자세한 내용은 여기를 클릭하세요 - https://www.databridgemarketresearch.com/reports/us-endoscope-reprocessing-market